 |
 |
- Search
| Korean J Helicobacter Up Gastrointest Res > Volume 21(3); 2021 > Article |
|

Abstract
Background/Aims
Subepithelial tumors (SETs) are small, mostly asymptomatic lesions with normal overlying mucosa, usually identified incidentally on endoscopy. The aim of this study was to evaluate the pathologic diagnosis of SETs, and to assess the diagnostic yield and impact of endoscopic submucosal dissection (ESD) biopsy on the management of patients with SETs.
Materials and Methods
We included 52 subepithelial lesions in this study during the study period. Inclusion criteria included size of the SET >2 cm, and a gastrointestinal stromal tumor (GIST) that cannot be excluded using EUS. We performed an endoscopic biopsy of each SET using the ESD technique.
Results
The mean diameter of the lesions was 24.15┬▒6.0 mm. The diagnostic yield of this method was 96.15%. Among the 52 participants, 45 were located in the stomach, four in the esophagus, and three in the duodenum. The pathologic diagnoses included: 17 leiomyomas, 13 GISTs, 11 ectopic pancreases, two carcinomas, two inflammatory fibroid polyps, two BrunnerŌĆÖs gland hyperplasia, two lipomas, one glomus tumor, and two remained undiagnosed. The mean duration of the procedure was 13.44┬▒2.41 minutes. Three complications were associated with the procedure.
Subepithelial tumors (SETs) are occasionally found in the gastrointestinal tract during screening endoscopy. They have normal overlying mucosa and are usually asymptomatic. However, SETs do have malignant potential, and it is therefore important to distinguish malignant from benign lesions. Several management plans have been proposed for patients with gastric SETs. For example, the National Comprehensive Cancer Network has recommended that all gastrointestinal stromal tumors (GISTs) of Ōēź20 mm in size be resected owing to their management potential owing to their malignant potential [1]. EUS can be used to preoperatively diagnose GISTs, although differential diagnosis on the basis of imaging alone is insufficient [2]. EUS-guided fine needle aspiration (FNA) increases diagnostic accuracy, but the results are quite variable. Lineartype EUS and EUS-FNA needles are needed for EUS-guided biopsy, and the technique has some limitations in small SET cases [3,4]. Therefore, we performed endoscopic biopsies of SETs using the endoscopic submucosal dissection (ESD) technique. The aim of this study is to evaluate pathologic confirmation in patients with SETs. And we also evaluate the diagnostic yield and impact of an ESD biopsy technique on the clinical management of patients with SETs.
A total of 52 patients (22 men, 30 women) who were scheduled to undergo EUS for gastric or duodenal SET between May 2010 and May 2015 were enrolled in the study. EUS images were examined for mass size, echogenicity, and invasion layer. All lesions eligible for participation based on EUS examination were >2 cm in diameter and were well circumscribed masses originating in the muscularis propria, submucosa or muscularis mucosal layer of the stomach. All patients had a normal complete blood count and prothrombin time. The ethics committee of hanyang university hospital approved the study protocol. Informed consent for the endoscopic procedure was obtained from each patient before the procedure.
Patients in whom a SET was identified during upper endoscopy and EUS were eligible to participate in this study. Endoscopic biopsy of SET was performed using a flex knife (Ji-In Corp, Ltd, Seoul, Korea), an IT 2-knife (Olympus, Tokyo, Japan), and a standard upper endoscope (GIF-H260; Olympus). All patients were given intravenous midazolam and pethidine before the procedure. All procedures were performed by one experienced endoscopist on an outpatient basis.
The ESD technique was performed as follows (Fig. 1). About 10 mL of epinephrine in hypertonic saline solution (dilution 1:1,000) was injected into the submucosa on the highest part of the lesion. Next, a 5-mm-diameter hole was created using a flex knife. Through this opening, the IT2-knife was introduced, and a round incision approximately 15 mm in diameter was made in the overlying mucosa using blend electrosurgical current. Then, submucosal dissection was performed with the IT2-knife. When the round mass was uncovered beneath the submucosal layer, we performed endoscopic biopsies five to six times by using forceps (radial Jaws 3, 2.8 mm outer diameter; Boston Scientific, Inc., Natick, MA, USA). After the procedure, closure was achieved with clipping.
Endoscopic histologic diagnosis of SET after the ESD technique was performed in the 52 patients enrolled in the study. The mean age of the 52 patients was 52.03┬▒13.35 years, and 22 were male. The clinical, endoscopic, and EUS study results of the patients are summarized in Table 1. The mean diameter of the SETs was 24.15┬▒6.0 mm. The diagnostic yield of this method was 96.15% (50/52). Of the 52 SETs, 45 were located in the stomach, four in the esophagus, and three in the duodenum. Their pathologic diagnoses were as follows: 17 leiomyomas, 13 GISTs, 11 ectopic pancreases, two carcinomas, two inflammatory fibroid polyps, two BrunnerŌĆÖs gland hyperplasias, two lipomas, one glomus tumor, and two remained undiagnosed. When our cases divided into two categories (benign vs. malignant), only 16 cases were malignant or malignant related lesions. The mean procedure time was 13.44┬▒2.41 minutes. There were three complications associated with procedure. Pneumomediastium was developed after the procedure in one patient. However, it was improved after conservative treatment. Patient whose biopsy result was glomus tumor showed active major bleeding during procedure. Endoscopic hemostasis was performed without problem. One patient presented with hematemesis 2 days after the procedure. Work-up revealed minimal bleeding at the procedure site. The bleeding stopped naturally without further endoscopic hemostasis. Surgical resection was performed in eight cases and all other cases are during regular follow up.
The present study suggests that deep biopsy using the ESD technique can provide key information for management of upper gastrointestinal SETs. These data also demonstrate that this modality can be safely and conveniently used. All procedures were performed on an outpatient basis.
Due to the widespread availability of high-resolution endoscopy, submucosal tumors (SMTs) arising in the upper gastrointestinal tract are increasingly being identified. When a SMT is encountered during upper endoscopy, the difficulty encountered in formulating a management plan lies in the uncertainty of the histopathology of the tumor [5,6].
Management plans for upper gastrointestinal SETs are determined using algorithms based on EUS images [7-9]. EUS has long been believed to improve the diagnostic precision of SETs. However, EUS morphologic features alone have limited specificity for the subtypes of SETs. EUS-FNA and EUS-guided trucut biopsy (EUS-TCB) are currently routinely performed. EUS-FNA biopsies can obtain specimens from desired exact location using with EUS image, but its exclusive instruments, technical difficulty and complications (accidental perforations, etc.) are disadvantages. Also obtain tissue cores is limited [10]. EUS-TCB biopsies have a higher diagnostic yield and can obtain tissue cores for immunohistochemical staining, but its efficacy is limited because of technical failure due to the stiffness of trucut needles [11,12]. The diagnostic yield of EUS-TCB ranged from 55% to 79% and that of EUS-FNA varied from 52% to 82% [12]. Furthermore, additional radial scopes, linear scopes, etc. are needed for EUS-FNA and fine-needle biopsy. These scopes and their accessories are expensive, however biopsy by using ESD can be performed with existing equipments. It is also difficult to obtain tissue samples using jumbo biopsy forceps and bite-on-bite techniques in SETs with normal overlying mucosa. Diagnostic yield of traditional unroofing biopsy of subepithelial lesions was 17~35% [13,14].
In Korea, as of 10 years ago, medical checkup programs are available to the whole nation. Therefore, asymptomatic SETs are occasionally diagnosed during routine endoscopy. Until now, there were no reliable guidelines about the management of SETs. Management plans vary depending on the physician. In our study, a surgical resection was considered for all participants, according to the size of the SET and the EUS findings. However, our pathologic results showed that only 16 of the 52 total cases were malignant lesions, which included GIST, adenocarcinoma, and glomus tumor. The development of ESD techniques have resulted in many reports about the effectiveness of ESD in large SETs [15-17]. The histopathologic results after ESD are mainly benign lesions such as leiomyoma or ectopic pancreas. Since the lesions that were thought to be SMT lesions were often different lesions, it is recommended revealing a pathological diagnosis before surgery.
In conclusion, our study suggests that histologic confirmation should be considered in upper gastrointestinal SETs before determining whether tumors should undergo long-term monitoring or surgical resection.
Acknowledgements
This work was supported by the Korean College of Helicobacter and Upper Gastrointestinal Research Foundation Grant.
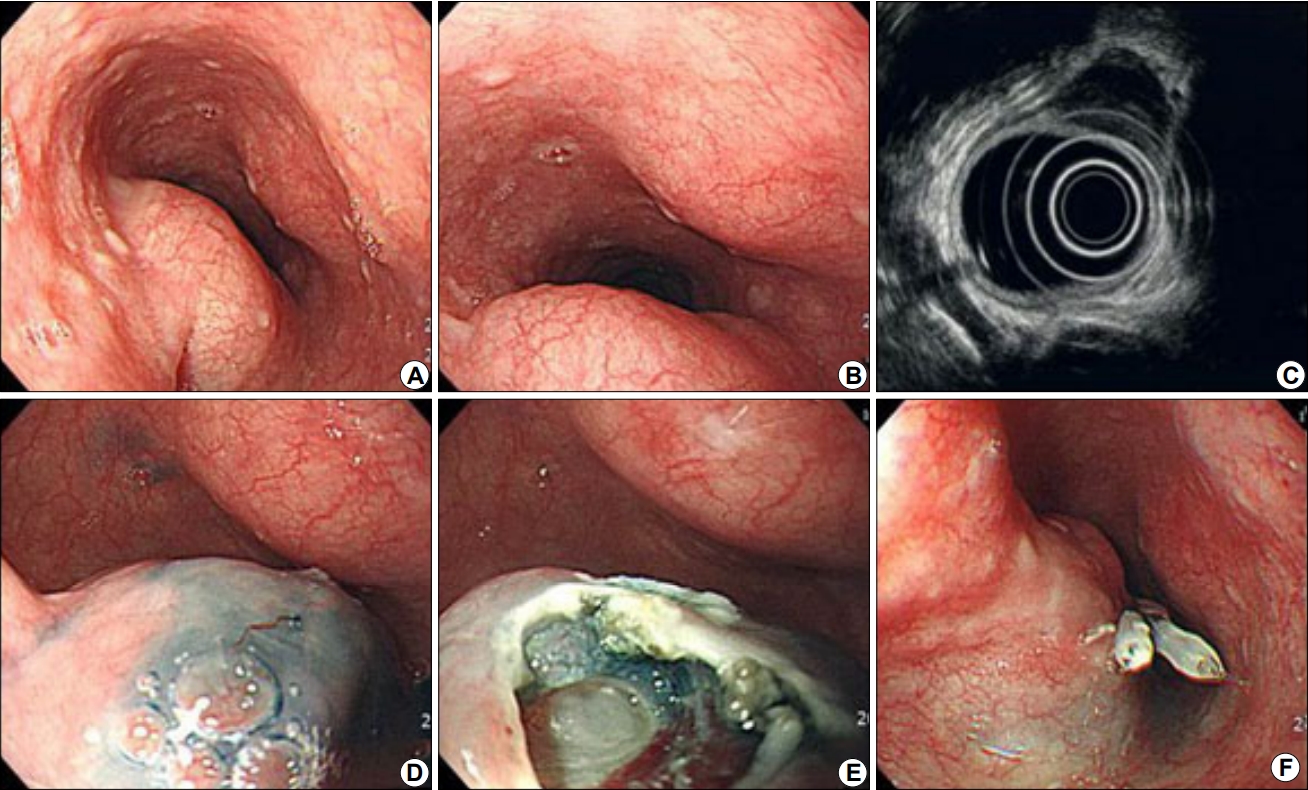
Fig.┬Ā1.
Endoscopic biopsy of an esophageal subepithelial tumor. (A, B) Subepithelial tumor in the esophagus. (C) Hypoechoic mass in the fourth layer. (D) Injection of hypertonic saline solution. (E) After the mucosal and submucosal layers were removed. (F) Closure was achieved with clipping.
Table┬Ā1.
Baseline Characteristics of the Participants
REFERENCES
1. Demetri GD, Benjamin RS, Blanke CD, et al. NCCN Task Force report: management of patients with gastrointestinal stromal tumor (GIST)--update of the NCCN clinical practice guidelines. J Natl Compr Canc Netw 2007;5 Suppl 2:S1ŌĆō29; quiz S30.


2. Hwang JH, Saunders MD, Rulyak SJ, Shaw S, Nietsch H, Kimmey MB. A prospective study comparing endoscopy and EUS in the evaluation of GI subepithelial masses. Gastrointest Endosc 2005;62:202ŌĆō208.


3. Hoda KM, Rodriguez SA, Faigel DO. EUS-guided sampling of suspected GI stromal tumors. Gastrointest Endosc 2009;69:1218ŌĆō1223.


4. Fern├Īndez-Esparrach G, Sendino O, Sol├® M, et al. Endoscopic ultrasound-guided fine-needle aspiration and trucut biopsy in the diagnosis of gastric stromal tumors: a randomized crossover study. Endoscopy 2010;42:292ŌĆō299.


5. Tae HJ, Lee HL, Lee KN, et al. Deep biopsy via endoscopic submucosal dissection in upper gastrointestinal subepithelial tumors: a prospective study. Endoscopy 2014;46:845ŌĆō850.



6. Lee HL, Kwon OW, Lee KN, et al. Endoscopic histologic diagnosis of gastric GI submucosal tumors via the endoscopic submucosal dissection technique. Gastrointest Endosc 2011;74:693ŌĆō695.


7. Kang YK, Kang HJ, Kim KM, et al. Clinical practice guideline for accurate diagnosis and effective treatment of gastrointestinal stromal tumor in Korea. Cancer Res Treat 2012;44:85ŌĆō96.




8. Hwang JH, Rulyak SD, Kimmey MB.; American Gastroenterological Association Institute. American Gastroenterological Association Institute technical review on the management of gastric subepithelial masses. Gastroenterology 2006;130:2217ŌĆō2228.


9. Eckardt AJ, Wassef W. Diagnosis of subepithelial tumors in the GI tract. Endoscopy, EUS, and histology: bronze, silver, and gold standard? Gastrointest Endosc 2005;62:209ŌĆō212.


10. Ito H, Inoue H, Ryozawa S, et al. Fine-needle aspiration biopsy and endoscopic ultrasound for pretreatment pathological diagnosis of gastric gastrointestinal stromal tumors. Gastroenterol Res Pract 2012;2012:139083.




11. Obuch J, Wani S. EUS-guided tissue acquisition in GI stromal tumors. Gastrointest Endosc 2017;86:516ŌĆō518.


12. Lee JH, Cho CJ, Park YS, et al. EUS-guided 22-gauge fine needle biopsy for the diagnosis of gastric subepithelial tumors larger than 2 cm. Scand J Gastroenterol 2016;51:486ŌĆō493.


13. Hedenbro JL, Ekelund M, Wetterberg P. Endoscopic diagnosis of submucosal gastric lesions. The results after routine endoscopy. Surg Endosc 1991;5:20ŌĆō23.



14. Cantor MJ, Davila RE, Faigel DO. Yield of tissue sampling for subepithelial lesions evaluated by EUS: a comparison between forceps biopsies and endoscopic submucosal resection. Gastrointest Endosc 2006;64:29ŌĆō34.


15. Li QL, Yao LQ, Zhou PH, et al. Submucosal tumors of the esophagogastric junction originating from the muscularis propria layer: a large study of endoscopic submucosal dissection (with video). Gastrointest Endosc 2012;75:1153ŌĆō1158.


- TOOLS
-
METRICS

-
- 0 Crossref
- 5,616 View
- 93 Download
- Related articles in Korean J Helicobacter Up Gastrointest Res
-
Common Gastric Subepithelial Tumors in Koreans2022 March;22(1)
A Case of Primary Aortoenteric Fistula Mimicking Duodenal Subepithelial Tumor2012 March;12(1)
A Case of Early Gastric Adenocarcinoma Resembling Subepithelial Tumor2013 March;13(1)
A Case of Gastric Calcifying Fibrous Tumor Presenting as a Subepithelial Tumor2013 December;13(4)






