Nutritional Strategies for Patients With Upper Gastrointestinal Cancers
Article information
Abstract
Patients with upper gastrointestinal tract cancers frequently develop severe malnutrition during treatment. Optimal nutritional management is imperative to enhance the efficacy of both chemotherapeutic and surgical interventions. In this article, various methods for nutritional screening and assessment, including a range of biochemical assays and decision-making frameworks relevant to clinical settings. We have described pre- and postoperative nutritional strategies and highlighted the established practical guidelines. Additionally, we investigated the key aspects of nutritional oversight during oncological treatment and emphasize the significance of a multidisciplinary approach that involves the services of physicians, dietitians, and nurses. We have outlined specific strategies to facilitate interprofessional collaboration in this setting.
INTRODUCTION
The upper gastrointestinal (GI) tract is an essential conduit for food intake. Tumors that involve this area predispose patients to dysphagia secondary to mechanical obstruction. Additionally, patients are at a high risk of malnutrition, which is often exacerbated by the adverse effects of anticancer therapies, such as chemotherapy and radiotherapy. A study in the United States has reported that 78.0% of patients diagnosed with esophageal cancer showed unintended weight loss of 10.0%– 15.0% at the time of diagnosis [1]. Furthermore, the prevalence of cachexia in patients with advanced or metastatic upper GI cancers, including esophageal and gastric cancers, ranks among the highest in cases of solid tumors [2,3].
Optimal nutritional status is crucial for improved treatment outcomes in patients with upper GI cancer during both chemotherapy and surgery. A low preoperative body mass index (BMI) is strongly correlated with poor post-gastrectomy outcomes and unfavorable long-term prognosis [4]. Moreover, preserving muscle mass through effective nutritional management both pre- and post-chemotherapy not only enhances treatment adherence but also decreases the risk of cancer recurrence [5]. Nutrition plays a significant role in the management of upper GI cancers; in this study, we describe methods for nutritional assessment and offer recommendations for nutritional care in the pre- and postoperative stages, as well as during anticancer treatment.
NUTRITIONAL EVALUATION
Nutritional evaluation comprises the following phases: 1) screening aimed at identification of patients at risk of malnutrition or those who are malnourished, and 2) assessment, which focuses on diagnosis of nutritional status through comprehensive analysis of patient data. During the screening phase, it is important to compare patient metrics (such as height, weight, weight loss, and dietary intake) with those recorded during previous visits.
Approximately 30 nutritional risk screening methods are available globally [6]. Among these, the Nutritional Risk Screening 2002 (NRS-2002) is specifically designed for hospitalized patients, the Malnutrition Universal Screening Tool is applicable across various clinical settings, and the Malnutrition Screening Tool is distinguished by its simplicity and user-friendliness [7]. The NRS-2002 is a representative instrument for evaluation of both existing nutritional deficiencies and the risk level in hospitalized patients and has been validated through retrospective analysis of data obtained from approximately 128 randomized clinical trials [8]. The evaluation criteria include weight loss, BMI, diminished dietary intake, and disease severity. Additional points are accorded to patients aged ≥70 years. A cumulative score of ≥3 necessitates nutritional support. It is recommended that surgery be postponed for 7–10 days to consider enteral or parenteral nutrition in patients with total scores >6, who are scheduled for an elective procedure [9].
Comprehensive evaluation of the patient’s nutritional status based on a broad range of data is essential in patients at risk for malnutrition based on nutritional screening. These data include a clinical history relevant to nutrition, medication history, weight fluctuations, dietary history, physical examination, anthropometric measurements, and biochemical tests. Although some of these factors overlap with the initial screening criteria, a more detailed dataset can be compiled to include elements such as dietary intake, energy levels, protein content, and micronutrients. Measurement of weight and BMI serve as simple but effective methods for evaluation of malnutrition. Repeat measurements, obtaining readings at consistent times and while wearing similar attire are important to ensure accurate metrics. BMI is measured by dividing weight (in kilograms) by the square of height (in meters) and serves as an indicator of chronic malnutrition. According to the World Health Organization criteria, based on the BMI, weight is classified ranging from underweight to normal weight, overweight, and obesity, with BMI <18.5 kg/m2 categorized as underweight [10]. However, exclusive reliance on weight and BMI is criticized because these parameters do not assess body composition, such as fat and muscle and cannot accurately determine unintentional weight loss or actual dietary intake.
The skinfold measurement technique, a simple method for evaluation of nutritional status in clinical settings, involves measurement of the circumference of the limbs (arms and legs) or the thickness of skinfolds at designated sites such as the biceps, triceps, subscapular, and suprailiac areas. Fat deposits at these sites indicate the overall body fat status and by extension, the total energy reserves. Bioelectrical impedance analysis (BIA) is a useful technique that involves the passage of an electrical current through the body to assess its composition. This method is useful to calculate the fat, muscle, and water ratio [11]. The electrical current flows readily through regions abundant in water and electrolytes, such as through blood and muscle and less so through fat, air, and bone; therefore, BIA is a valuable assessment tool.
Although biochemical tests serve as useful indicators for nutritional evaluation, reliance on a single biomarker for evaluation or monitoring is inadvisable [12]. Basic tests such as a complete blood count, total lymphocyte count, lipid profile, electrolytes, and liver function tests can yield useful information regarding an individual’s nutritional status. Serum albumin and prealbumin levels are commonly used to identify patients at risk of malnutrition. The Geriatric Nutritional Risk Index (GNRI) is a scoring system that leverages these markers [13]. The GNRI is calculated based on total albumin levels, weight, and ideal body weight and has been utilized to evaluate the pre- and postoperative prognosis of patients diagnosed with early-stage gastric cancer. GNRI scores <96 indicate poor outcomes [14]. However, the interpretation of these test results should be contextual because these may be affected by inflammatory biomarkers such as C-reactive protein levels and often show low sensitivity and specificity.
The Global Leadership Initiative on Malnutrition (GLIM), a novel malnutrition assessment tool, aims to standardize diagnosis of malnutrition in clinical practice and is endorsed by leading clinical nutrition societies, including the American Society for Parenteral and Enteral Nutrition, European Society for Clinical Nutrition and Metabolism (ESPEN), Federación Latinoamericana de Terapia Nutricional, Nutrición Clínica y Metabolismo, and the Parenteral and Enteral Nutrition Society of Asia [15,16]. GLIM recommends a two-step approach comprising initial screening to identify patients “at risk” using any validated tool, followed by comprehensive assessment for diagnosis and grading of malnutrition severity. Diagnostic criteria for malnutrition include both phenotypic and etiologic factors [16]. Phenotypic criteria include involuntary weight loss, low BMI, and diminished muscle mass, and etiologic criteria include reduced food intake or assimilation and inflammation or disease burden. At least one phenotypic and one etiological criterion must be fulfilled to definitively diagnose malnutrition.
PERIOPERATIVE NUTRITIONAL MANAGEMENT
Surgery remains the most definitive and effective treatment for localized esophageal and gastric cancers. The key outcome indicators associated with malnutrition (pre- and postoperatively) include postoperative complications, length of hospital stay, quality of life, and long-term survival. However, patients with upper GI cancer show a significantly high risk of nutritional imbalance and weight loss both pre- and postoperatively, which subsequently leads to a high incidence of adverse outcomes in these indicators.
The following mechanisms contribute to severe malnutrition in patients who undergo surgery for upper GI cancer: 1) surgical bypass involving the esophagus, stomach, and duodenum may result in malabsorption. This is particularly relevant in patients who undergo operations involving duodenal bypass; malabsorption is more frequent after gastroduodenal anastomosis than after gastrojejunal anastomosis. 2) Alterations in gastric volume may result in reduced food intake. 3) Vagotomy-induced reduction in motility and fundal capacity also contribute to malnutrition.
The gut-brain axis facilitates transmission of nutritional information from the GI tract to the brain; the vagus nerve plays a pivotal role in transporting these GI stimuli. Functions of the myenteric nerve fibers differ slightly between the proximal and distal parts of the stomach. The proximal section induces smooth muscle relaxation, which enables food storage, whereas the distal section facilitates grinding and mixing of food. Gastric surgery can result in various degrees of myenteric nerve injury, which can affect gastric motility and secretion. Conventional gastrectomy is usually associated with more pronounced injury to the proximal myenteric nerves, which results in inhibition of relaxation and reduced storage capacity [17]. Injury to the distal myenteric nerves can precipitate gastric paralysis, with consequent functional gastric outlet obstruction for solid foods.
Several studies have reported that improved perioperative nutrition is associated with favorable postoperative outcomes. In a study performed by Kim et al. [18], 510 patients who underwent gastric cancer surgery were stratified based on BMI categories (low ≤18.5 kg/m2, normal, and high ≥25.0 kg/m2), and the authors observed that low preoperative BMI adversely affected survival rates in patients with stage I/II disease and increased the risk of severe postoperative complications in patients with stage III/IV cancer. Conversely, Lee et al. [19] investigated 1909 patients who underwent gastric cancer surgery, focusing on the association between preoperative BMI and postoperative survival outcomes. The authors observed that overall, disease-specific, and recurrence-free survival were longer in the high-BMI group than in the low- and normal-BMI groups. Therefore, evidence suggests that both pre- and postoperative BMI are positively correlated with improved clinical outcomes after surgery.
In 2021, ESPEN revised the practical guidelines for clinical nutrition in surgical settings. These updated guidelines offer evidence-based recommendations to optimize nutritional support rendered to patients who undergo surgery to improve their postoperative outcomes. According to these guidelines, patients should undergo preoperative nutritional screening using the NRS-2002, and it is recommended that surgery be postponed for 7–10 days, and during this interval, enteral or parenteral nutrition be considered in those with scores ≥6. Decisions between these two nutritional approaches should consider the various risks associated with parenteral nutrition. Patients without gastric emptying issues can safely consume a carbohydraterich drink up to 2 hours preoperatively because the stomach is nearly empty 2 hours after ingestion of food/drink, and there is no surgical risk. This approach minimizes postoperative insulin resistance and its complications. Depending on the NRS-2002 score, oral nutritional supplements and immunonutrition may also be considered preoperatively [9].
Postoperative nutritional management is based on the Enhanced Recovery After Surgery guidelines. Early initiation of an enteral liquid diet via a feeding jejunostomy or nasojejunal/nasoduodenal tubes, with a targeted nutritional rate is strongly recommended for 3–6 days postoperatively in patients who undergo esophagectomy (Table 1) [20]. Exclusive parenteral nutrition is limited to patients in whom the GI tract is either nonfunctional secondary to prolonged obstruction or absent owing to conditions such as intra-abdominal sepsis or fistulas.
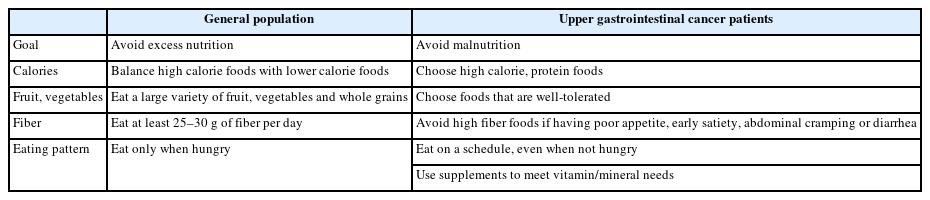
Initiation of fluids and food is recommended on the first post-operative day (POD) to eliminate the need for “nothing by mouth” time in patients who undergo total gastrectomy [21]. GI function usually recovers rapidly postoperatively, and routine nutritional support is largely redundant. However, individualized nutritional support via tube or parenteral feeding is advised for patients who are unable to meet at least 60.0% of their daily nutritional needs by POD 6 [9]. Routine decompression via nasogastric or nasojejunal tubes is usually not recommended considering the potential risk of pulmonary complications. Contrary to standard dietary guidelines, which aim to prevent excessive nutrient intake, efforts must focus on prevention of malnutrition during the postoperative phase of upper GI cancer. Patients should be instructed to consistently consume small and frequent meals even if not hungry, and a high-protein, lowfiber diet is recommended (Table 2).
NUTRITIONAL MANAGEMENT DURING ANTICANCER TREATMENT
Patients with cachexia, particularly those with upper GI cancers tend to have reduced tolerance to chemotherapy and radiation, which subsequently negatively affects their quality of life. A study that focused on the prevalence of cachexia across different cancer types reported that 76.4% of patients with upper GI cancers develop cachexia; this rate is significantly higher than that observed in patients with head and neck cancers (50.9%) or in those with hematologic cancers (50.6%) [3]. Upper GI cancers are often associated with nutrition impact symptoms (NIS) such as anorexia, nausea, and malabsorption, which contribute to poor overall survival. Studies that have investigated clinical and nutritional factors that affect overall survival have observed that patients with NIS ≥3 show significantly shorter overall survival [22]. Additionally, chemotherapy induced adverse effects can precipitate severe malnutrition; a study has reported a significant decline in both macro and micronutrient intake among all patients who followed their chemotherapy regimen [23].
The primary objectives of nutritional support in patients with cancer should focus on weight and muscle maintenance, improved treatment response, and minimizing fatigue. The ESPEN guidelines for nutrition in patients with cancer recommend a high protein intake (1.2–1.5 g/kg/day) together with increased physical activity [24]. A systematic review of existing literature observed that most studies corroborated the benefits of branched-chain amino acid supplementation, which improved liver function, prevented abdominal fluid accumulation, promoted better albumin metabolism, inhibited cancer growth, improved handgrip strength, reduced insulin resistance, and improved survival rates in patients with solid tumors such as esophageal cancer [5].
Glutamine supplementation is widely recognized for its effectiveness in alleviation of radiation-induced oral sores and diarrhea. These products can be administered orally, as a mouthwash or intravenously. Intravenously administered glutamine reduces the frequency, severity, and duration of symptoms in patients who develop radiation-induced mucositis following administration of 5-fluorouracil (5-FU)/cisplatin chemotherapy [25]. However, glutamine is associated with high rates of tumor relapse in patients who undergo hematopoietic stem cell transplantation; therefore, caution is warranted in patients who receive these supplements. Per ESPEN guidelines, existing clinical data are inconsistent to support glutamine use for management of radiation-induced intestinal complications, oral sores, and esophageal inflammation [24].
Chemotherapy-induced nausea and vomiting (CINV) is among the most common symptoms associated with chemotherapy. CINV negatively affects patients’ quality of life and can interrupt treatment regimens. CINV involves the activity of various receptors and neurotransmitters, such as serotonin and neurokinin-1 receptors and is triggered by activation of the vomiting center in the central nervous system. Chemotherapeutic agents are classified into high, moderate, low, and minimal categories based on their emetic risk. Prophylactic interventions are essential for agents with high and moderate emetic risk, whereas symptom-based treatment is sufficient for agents with low and minimal risk. Excluding cisplatin, which is classified as a high-risk drug, most medications used to treat GI cancers are of moderate (irinotecan, oxaliplatin, carboplatin) or low (cetuximab, gemcitabine, 5-FU, nab-paclitaxel) emetic risk [26]. Furthermore, it is imperative to assess the distinct attributes of the chemotherapeutic agents used, as well as patientspecific risk factors, including a history of motion sickness, age <60 years, previous episodes of CINV, prior chemotherapy exposure, and inadequate sleep before chemotherapy [27]. These variables more accurately predict the risk of CINV.
ROLE OF A MULTIDISCIPLINARY APPROACH
Considering the complex health challenges observed in patients with upper GI cancer, which range from nutritional concerns to physical limitations and emotional stress, a multidisciplinary team-based approach is indispensable to address individual nutritional needs. The primary objective of this approach is to improve patients’ nutritional intake, encourage physical activity, build physical strength, and promote mental well-being. Interprofessional coordination between various healthcare domains is necessary to deliver effective patient care consistent with the needs of hospitalized patients or of those receiving home-based care.
Successful implementation of a multidisciplinary approach necessitates explicit delineation of roles and interdepartmental collaboration. Effective communication and implementation of information sharing protocols are essential. In many instances, central coordination of these multifaceted efforts necessitates both clinical leadership and administrative support. The Interdisciplinary Alliance to Advance Patient Nutrition is instrumental in providing practical recommendations to achieve such cooperation [28]. This alliance outlines the roles of physicians, dietitians, nurses, and hospital administrators in collectively meeting six nutritional objectives through teamwork. Uniform and consistent methods to measure and share data are essential throughout the care process, from admission until discharge and even extending to post-discharge follow-up.
CONCLUSION
Nutritional management plays a key role in determining treatment outcomes in patients with upper GI cancers. Implementation of a multidisciplinary approach complemented by comprehensive nutritional assessment and an effective care strategy are essential to improve both the long-term prognosis and effectiveness of therapeutic interventions.
Notes
Availability of Data and Material
The datasets generated or analyzed during the study are available from the corresponding author on reasonable request.
Conflicts of Interest
The author has no financial conflicts of interest.
Funding Statement
None
Acknowledgements
None