Comparison of Helicobacter pylori Eradication Rates Using Standard Triple Therapy and Sequential Therapy
Article information
Abstract
Objectives
The incidence of treatment failures following standard triple therapy (STT) for Helicobacter pylori eradication (HPE) has reached an unacceptable level. Sequential therapy (SQ) has emerged as a promising approach to counteract the escalation of antibiotic resistance. In this study, we used a chronological cohort dataset to conduct a comparative analysis of the eradication rates, compliance, and adverse events associated with the 7-day STT and SQ.
Methods
A total of 789 patients underwent HPE treatment at Asan Medical Center between July 2013 and August 2017. Among them, 378 received a 7-day STT and 411 received a 10-day SQ. Baseline clinical data and treatment parameters were compared between the two treatment groups.
Results
SQ demonstrated an eradication rate of 84.7% (348/411), which was superior to that of the 7-day STT (74.1%; p<0.001). The incidence of adverse events was also higher in the SQ group than in the STT group (17.5% vs. 11.1%; p=0.01). Nonetheless, treatment compliance was not significantly different between the groups (98.1% [SQ] vs. 96.8% [STT]; p=0.38). Among the patients undergoing second-line eradication, the SQ group displayed a lower eradication rate than the STT group (77.8% vs. 92.4%; p=0.028). Notably, the overall eradication rate did not differ significantly between the two groups (98.3% [STT] vs. 97.4% [SQ]; p=0.56).
Conclusions
SQ exhibited superior efficacy compared with the 7-day STT as a first-line H. pylori treatment. Thus, SQ holds potential to serve as the replacement for the 7-day STT in treatment-naïve patients.
INTRODUCTION
Helicobacter pylori is an established etiological factor for peptic ulcer disease, gastric mucosa-associated lymphoid tissue lymphoma, and gastric cancer[1]. The bacterium is known to have affected approximately 50% of the global population, with a notably higher prevalence in developing countries [1,2]. Standard triple therapy (STT), comprising a proton pump inhibitor (PPI), clarithromycin, and amoxicillin, has traditionally been considered as the gold standard regimen for H. pylori eradication (HPE) in many regions of the world [3-5]. However, suboptimal eradication rates and instances of treatment failure have progressively escalated, reaching an unacceptable level [6-10]. In response, sequential therapy (SQ) has emerged as a promising approach to counteract the elevated levels of antibiotic resistance[11]. This novel regimen involves a distinctive sequence of antibiotic administration: a 5-day course of amoxicillin followed by 5-day course of clarithromycin and metronidazole, supplemented by 10 consecutive days of PPI intake. Following its initial implementation in Italy, several studies have reported higher eradication rates for SQ than that of the 7-day STT [12-16]. However, existing data present conflicting results regarding the efficacy of eradication rates, reporting no significant difference between the two treatment regimens [17,18]. Therefore, we conducted a retrospective analysis of patients who underwent H. pylori treatment between July 2013 and August 2017 at a single tertiary hospital. In this study, we aimed to provide insights into the outcomes related to eradication rates and adverse events associated with the use of the 7-day STT and SQ.
METHODS
Patients
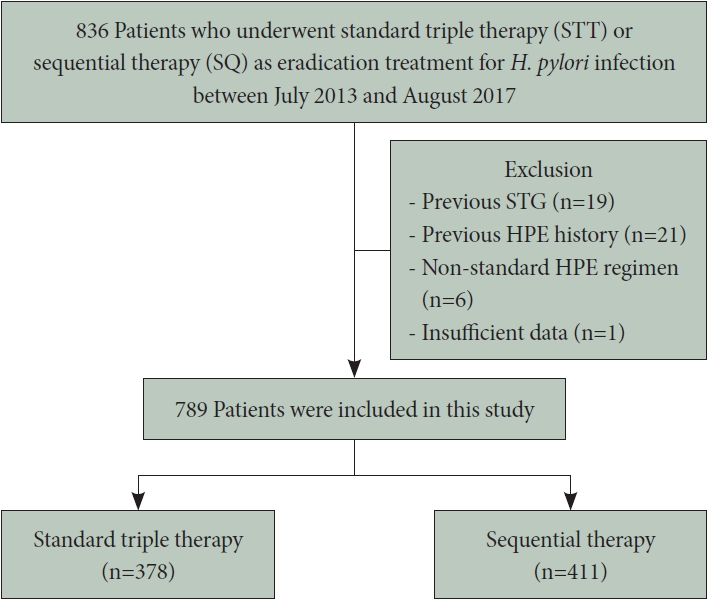
We retrospectively analyzed consecutive data collected from patients who underwent eradication therapy for H. pylori infection and subsequent 13C-urea breath tests (UBT) at Asan Medical Center between July 2013 and August 2017 (Fig. 1). The study included only patients who had not previously received HPE treatment and for whom eradication was performed under the direction of a single physician. Among the initial 836 patients who underwent STT or SQ eradication therapy, 47 were excluded from the analysis due to a history of gastric surgery (n=19), previous eradication therapy (n=21), non-standard regimen use (n=6), or insufficient data (n=1). Among the remaining 789 patients, 378 received STT for 7 days as their first-line HPE treatment (STT group) and 411 received SQ for 10 days (SQ group). For these patients, clinical data including eradication indications, eradication rates, medication compliance, treatment-related adverse events, and family history of gastric cancer were retrospectively reviewed. Patients were requested to return at the end of therapy for an assessment of compliance and adverse events.
Definition of H. pylori infection and confirmation of eradication
All patients underwent upper gastrointestinal endoscopy at baseline, and H. pylori infection was determined based on positive results from either a UBT or rapid urease test (RUT) results. After treatment, all patients underwent a 13C-UBT, which was conducted at least 4 weeks after discontinuing PPI. RUT was performed using the Campylobacter-like organism test card (Kimberly-Clark, Roswell, NM, USA) or the CKD Bio Hp kit (Chong Kun Dang, Seoul, Korea). The 13C-UBT was performed using a 13CO2-infrared spectrophotometry analyzer (POC One; Otsuka Pharmaceutical, Osaka, Japan) to detect urease activity as a surrogate marker of H. pylori infection. Patients were instructed to fast for a minimum of 4 h before the tests. After obtaining an initial singlebreath sample, the patients were instructed to ingest a 100 mg 13C -labeled urea capsule (Otsuka Pharmaceutical) along with 100 mL of water. Following a 5-minute period in the left decubitus position and a 15-minute interval in a seated position, the patients exhaled into a new breath-collection balloon. The ratio of 13CO2 to 12CO2 (δ13CO2) in the two breath samples was measured.
Therapeutic regimen
The STT regimen comprised lansoprazole (30 mg), amoxicillin (1000 mg), and clarithromycin (500 mg), administered twice daily for 7 days. The SQ regimen consisted of lansoprazole (30 mg) and amoxicillin (1000 mg) administered twice daily for 5 days, followed by a combination of lansoprazole (30 mg), clarithromycin (500 mg), and metronidazole (500 mg) administered twice daily for 5 days. The UBT was conducted at least 4 weeks after the completion of eradication therapy. Patients who required PPIs for symptomatic relief or treatment of iatrogenic peptic ulcers after endoscopic resection for gastric adenoma or early gastric cancer were instructed to discontinue PPI intake at least 2 weeks prior to the UBT. Patients for whom eradication failed were recommended to undergo quadruple therapy consisting of lansoprazole (30 mg twice daily), tetracycline (500 mg four times daily), metronidazole (500 mg twice daily), and bismuth (120 mg twice daily) for 14 days.
Assessment of HPE rate
Compliance was defined as consumption of >90% of the prescribed medications. Adverse events during treatment were evaluated through personal interviews with the physician. The eradication rate was analyzed only in compliant patients. The overall eradication rate was defined as the percentage of successful eradications achieved using either the first- and/or second-line regimen(s) and was calculated using patients for whom second-line regimen results were available.
Statistical analysis
Baseline variables are presented as numbers (percentages) and means (standard deviations). Continuous variables were compared using the Student’s t-test, and categorical variables were assessed using the chi-square test or Fisher’s exact test. All p-values were two-sided, and a p-value <0.05 was considered significant. All statistical analyses were performed using IBM SPSS version 21.0 software (IBM Corp., Armonk, NY, USA).
Ethical approval
Ethical approval for data acquisition was obtained from the Institutional Review Board of Asan Medical Center (No. 2015-0838), and the requirement for informed consent was waived because the data were collected retrospectively.
RESULTS
Baseline characteristics of the patients
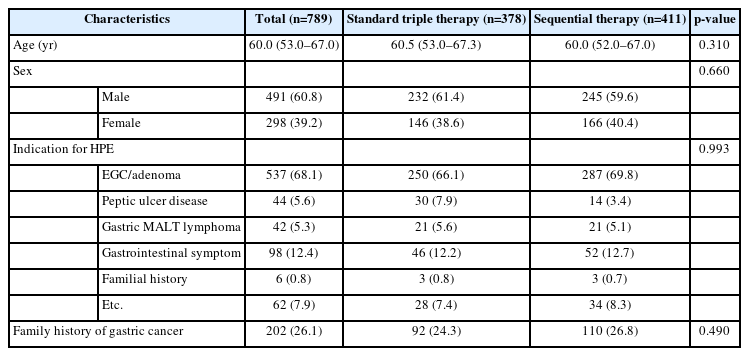
Table 1 presents the baseline demographic and clinical characteristics of the included patients. The median age and sex distribution in the SQ group were comparable to those in the STT group. The most common indication for eradication therapy in both groups was endoscopic resection of early gastric cancer or adenoma, accounting for 66.1% and 69.8% of patients in the STT and SQ groups, respectively. No significant disparity was observed in the indications for eradication therapy between the two groups.
HPE rates
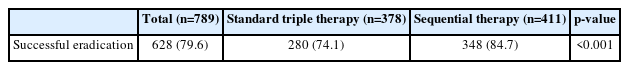
Fig. 1 illustrates flowchart depicting patient inclusion in the study. Among the 378 patients in the STT group, 4 (1.1%) were non-compliant, and compliance data were absent for 8 (2.1%) patients. In the SQ group (n=411), 4 (1.0%) patients exhibited <90% compliance, and data were unavailable for another 4 patients (1.0%). Treatment compliance was not significantly different between the two groups (STT: 96.8% vs. SQ: 98.1%; p= 0.380). Notably, the eradication rate in the SQ group surpassed that in the STT group (84.7% vs. 74.1%, respectively; p<0.001) (Table 2).
Adverse events during eradication therapy
Table 3 outlines the adverse events reported during HPE. The incidence of adverse events was significantly higher in the SQ group than that in the STT group (16.8% vs. 11.4%, respectively; p=0.010). The most prevalent adverse events in both groups were abdominal discomfort and pain (STT: 5.6%; SQ: 6.3%). The distribution of specific adverse events was comparable between the two groups. All patients who experienced adverse events exhibited mild symptoms, and none required additional treatment or discontinuation of HPE therapy due to these events.
Quadruple therapy outcomes in treatment-failure patients
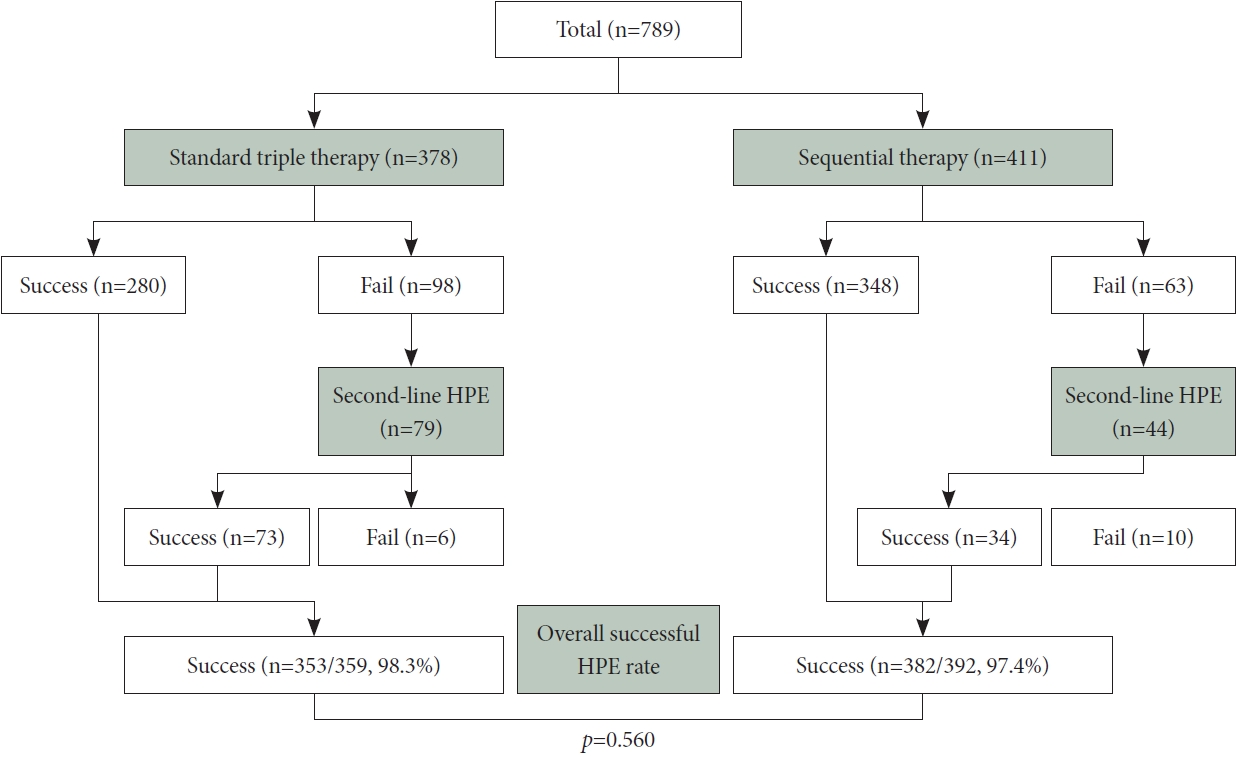
In the STT group, eradication therapy was ineffective in 98 patients, whereas the SQ group had 63 cases of treatment failure. Among the 98 treatment-failure patients in the STT group, 79 received quadruple therapy as a second-line regimen; among them, 73 achieved successful eradication. In contrast, the SQ group displayed a notably lower eradication rate (77.3% vs. 92.4%, respectively; p=0.028) (Fig. 2). The overall eradication rates for both first- and second-line HPE regimens were calculated for the STT and SQ groups, yielding results of 98.3% (353/359) and 97.4% (382/392) (p=0.560) (Fig. 3).
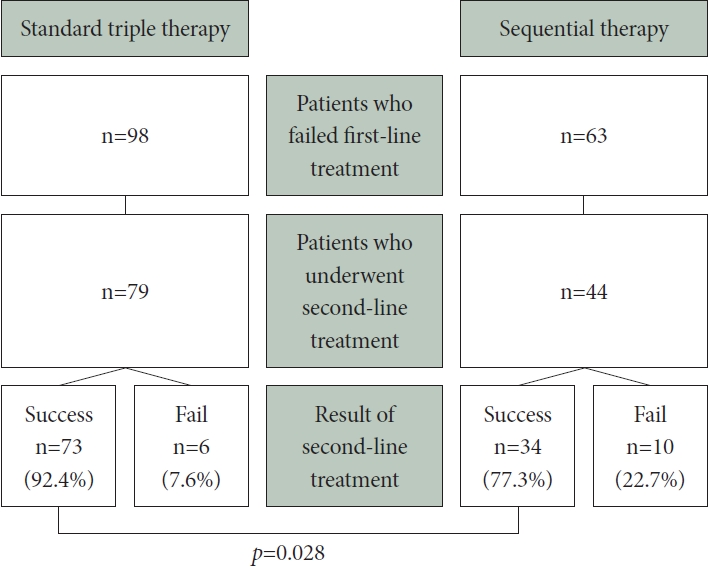
Results of the second-line Helicobacter pylori eradication in patients with first-line treatment failure. A summary of the outcomes of second-line Helicobacter pylori eradication (HPE) treatment based on the initial first-line HPE regimen for patients who experienced first-line therapy failure. Notably, the success rate in the sequential therapy group is significantly lower than that in the triple therapy group (p=0.028).
DISCUSSION
In this study, we compared the eradication efficacies of the 7-day STT and SQ, which are both representative first-line treatment regimens for HPE in Korea. Our findings revealed that SQ had a significantly higher eradication rate than that of the 7-day STT, and it did not exhibit a decrease in compliance when used as a first-line treatment for H. pylori infection. STT has historically been the recommended as a primary treatment regimen in multiple guidelines, including the Guidelines for the Diagnosis and Treatment of Helicobacter pylori Infection published in 2013 by the Korean College of Helicobacter and Upper Gastrointestinal Research [19]. However, numerous reports have indicated a global decline in the eradication rate (<80%) associated with STT [6,8,9]. Since its introduction into the Korean market in the 1990s, clarithromycin has been the cornerstone of HPE therapy, administered in combination with a PPI to counteract the acidic gastric environment [20]. Nevertheless, a surge in clarithromycin-resistant H. pylori strains has emerged as a key contributor to treatment failures. Recent studies have identified that clarithromycin resistance rates in Korea range from 17.8% to 31.0% [21,22]. Consequently, the latest revision of the Helicobacter pylori infection guidelines in Korea recommends not only a 14-day triple therapy but also non-bismuth quadruple therapy, including SQ, as the first-line HPE regimen [3].
The superiority of SQ has been demonstrated in studies conducted across Asian and European countries since its introduction by Zullo et al. [11] in 2000 [11,23-26]. Eradication rates may vary by geographical region owing to the differences in antibiotic resistance patterns; however, in the majority of these studies, SQ consistently exhibited higher eradication rates (81.8%–94.2% in an intention-to-treat [ITT] analysis) than that of the standard 7-day STT (70.0%–74.3% in an ITT analysis). In line with these prior investigations, our study demonstrated an eradication rate of 84.7% for the SQ group, surpassing the rate observed for the STT group. The theoretical advantages of SQ include the administration of amoxicillin before that of clarithromycin and metronidazole during the first 5 days, which may lower H. pylori density in the stomach, potentially enhancing the efficacy of clarithromycin and metronidazole. Additionally, the action of amoxicillin as a cell wall synthesis inhibitor could potentially explain the inhibition of efflux channel production, a known mechanism of clarithromycin resistance [27].
Our study revealed that patients in the SQ group experienced more antibiotic-related adverse events (16.8%) than those of patients in the STT group (11.4%); abdominal discomfort and pain were the most common adverse events in both the groups. However, all adverse events were mild in nature, and none of the patients required hospitalization or therapy discontinuation owing to symptoms. Considering the risk-benefit balance between the therapeutic efficacy and complication rates of the two regimens, SQ remains a viable and effective treatment approach.
Although the first-line treatment eradication rate was notably higher in the SQ group than in the STT group, the second-line eradication rate (involving bismuth-based quadruple therapy for patients who failed the initial treatment) was lower in the SQ group. This may be attributed to the emergence of metronidazole resistance among patients who received SQ. Nevertheless, given the limited number of patients undergoing second-line eradication compared with the total cohort, this result may be subjected to statistical errors due to the small sample size. Furthermore, this disparity was mitigated when the first- and second-line treatment results were combined to calculate the overall eradication efficacy. To address this issue, future studies should encompass larger patient populations and include antibiotic susceptibility testing.
The limitations of our study included the retrospective study design and data collection at a single institution. Moreover, the absence of antibiotic resistance data hindered a comprehensive understanding of the exact mechanisms underlying the higher eradication rate in the SQ group than in the STT group. Additionally, current guidelines suggest a 14-day triple therapy in the absence of antibiotic susceptibility testing, necessitating further research for patients treated with 14-day triple therapy and SQ. Finally, further investigations should examine whether the efficacy and cost-effectiveness of SQ are superior to those of other options, such as concomitant therapy or bismuth quadruple therapy.
In conclusion, our findings supported the superiority of SQ over a 7-day STT as a first-line treatment for H. pylori infections. SQ has the potential to serve as a substitute for 7-day STT in treatment-naïve patients, particularly in medical settings where antibiotic susceptibility testing may be limited.
Notes
Availability of Data and Material
The datasets generated or analyzed during the study are available from the corresponding author on reasonable request.
Conflicts of Interest
The authors have no financial conflicts of interest.
Funding Statement
None
Authors’ Contribution
Conceptualization: Yuri Kim, Hwoon-Yong Jung. Data curation: all authors. Formal analysis: Yuri Kim. Investigation: Yuri Kim. Methodology: Hwoon-Yong Jung. Project administration: Hwoon-Yong Jung. Supervision: Hwoon-Yong Jung. Validation: all authors. Visualization: Yuri Kim. Writing—original draft: Yuri Kim. Writing—review & editing: Yuri Kim. Approval of final manuscript: all authors.
Acknowledgements
None