|
 |
- Search
| Korean J Helicobacter Up Gastrointest Res > Volume 21(2); 2021 > Article |
|

Abstract
Background/Aims
Proton pump inhibitors (PPIs) are widely used to treat several acid-related gastrointestinal disorders. This study aimed to investigate the risk of dementia in patients taking PPIs.
Materials and Methods
A systematic review was conducted to evaluate the correlation between PPIs and dementia. The methodological quality of the included studies was evaluated using the Risk of Bias Assessment tool for non-randomized studies. Publication bias was assessed.
Results
A total of 12 nested, case-control, and cohort studies were identified and analyzed. We obtained hazard ratios (HRs) from five studies and performed a meta-analysis. The meta-analysis of four cohort studies and one nested case-control study showed no association between PPIs and dementia (HR, 1.165; 95% CI, 0.912~1.488; P=0.222). Sensitivity analysis revealed consistent results. No publication bias was detected.
Proton pump inhibitors (PPIs) are a class of medication prescribed for healing and preventing esophagitis and peptic ulcer disease [1,2]. PPIs are perceived to have a favorable side effect profile. They are often prescribed not only for established acid-related upper gastrointestinal disease, but also for nonspecific upper gastrointestinal symptoms empirically [3,4]. As a result, PPIs are the most widely used class of drugs prescribed long-term among all clinical medicine [5]. Approximately 8~10% of adults have been prescribed PPIs in the past 30 days [5]. PPI use is particulary prevalent in the elderly; people over the age of 60 years are 3.5 times more likely to be using PPIs than those under 60 years [6].
Numerous studies have raised doubts about the long term safety of PPI use [7,8]. PPI has been shown to increase the synthesis of certain amyloid-├¤ protein, which is also elaborated in patients with AlzheimerŌĆÖs disease (AD) [9]. PPIs has also been associated with vitamin B12 deficiency which may contribute towards the development of dementia [10]. Recently two studies from Germany reported a potential association between PPI use and increased risk of dementia [11,12]. This is particularly concerning because PPIs are more frequently used among older individuals where the risk of dementia is high. A previous systematic review of 11 studies exploring the association of PPI use and dementia could not reach definitive conclusions due to limitations of methodological issues [13]. Recently, the results of several epidemiologic studies have pointed toward a null association between PPI use and dementia. Therefore, we performed this systematic review and meta-analysis to further clarify the association between PPI use and dementia.
This systematic review with meta-analysis was conducted using a priori protocol (PROSPERO registration number: CRD42018102583). It fully adhered to the principles of the Preferred Reporting Items for Systematic reviews and Meta-Analyses checklist [14].
MEDLINE (through PubMed), the Cochrane library, and Embase were searched using common keywords associated with PPIs and dementia (from inception to May 2018). Medical Subject Heading or Emtree keywords were selected to search electronic databases. Titles and abstracts of all included studies were reviewed to exclude irrelevant publications. Full-text reviews were performed to determine whether inclusion criteria were satisfied in remaining studies. Reference lists from articles selected via electronic searches were manually searched to obtain further relevant studies. Article selection was performed independently by two authors (K.T.Y. and J.S.K.). Differing decisions were resolved by consensus.
We included studies that met the following criteria: 1) studies designed to evaluate the effect of PPI use and association with dementia; 2) studies of human subjects; 3) publications in English; and 4) full-text publications. Studies that met all four inclusion criteria were sought and selected. Exclusion criteria were as follows: 1) review articles; 2) guidelines or consensus documents or expert position papers; 3) comments, letters, brief reports, proceedings, protocol studies; 4) case reports; 5) publications with incomplete data; or 6) meta-analysis articles.
Two review authors (K.T.Y. and J.S.K.) independently extracted data from included studies using a common pre-data extraction form. They resolved any discordance in assessments via discussion. If any clarification of data was necessary, more information was requested from investigators. The primary outcome of interest was the effect of PPIs on the development of dementia. Ratios were extracted and evaluated from studies using hazard ratio (HR), whenever possible. Subgroup analyses were also performed to identify the source of heterogeneity based on multiple modifiers identified during the systematic review and to confirm the robustness of the main result. These modifiers included study location, study type, study quality, sex, and duration of medication use.
Methodological quality of included publications was assessed using the Risk of Bias Assessment tool for non-randomized studies (RoBANS) [15]. The RoBANS tool contains six domains. It is a validated tool that is reliable and feasible for assessing the methodological quality of non-randomized studies. Two authors (K.T.Y. and J.S.K.) independently assessed the methodological quality of the included studies. Any disagreement between the authors was resolved by discussion. Review Manager version 5.3.3 (Revman for Windows 7; the Nordic Cochrane Centre, Copenhagen, Denmark) was used to generate the summary of RoBANS results.
Comprehensive Meta-Analysis software (version 2; Biostat Inc., Englewood, NJ, USA) was used for this meta-analysis. We calculated HRs with 95% CIs using 2├Ś2 tables from the original articles to evaluate the effect of PPIs on the development of dementia whenever possible. Heterogeneity was determined using the I2 test developed by Higgins to measure the percentage of total variation across studies [16]. I2 was calculated as follows: I2 (%)=100├Ś(Q df)/Q, where Q was CochraneŌĆÖs heterogeneity statistic and df was the degree of freedom. Negative values for I2 were set to zero. An I2 value over 50% indicated substantial heterogeneity (range, 0~100%). Pooled-effect sizes with 95% CIs were calculated using a random-effects model and the method of DerSimonian and Laird [17] due to methodological heterogeneity. These results were confirmed by the I2 test. Significance was set at PŌēż0.05. Publication bias was evaluated using BeggŌĆÖs funnel plot, EggerŌĆÖs test of the intercept, and Begg and MazumdarŌĆÖs rank correlation test [18-20]. We performed cumulative meta-analysis (defined as the performance of an updated meta-analysis every time a new trial appears) for evaluating the results as a continuum [21]. These techniques make it possible to study trends in good and bad effects and to pinpoint the first time a difference in outcome between treatment and control groups becomes statistically significant at a chosen level. Also, to evaluate the influence of each study on the overall effect size, sensitivity analysis was conducted using the one-study remove approach.
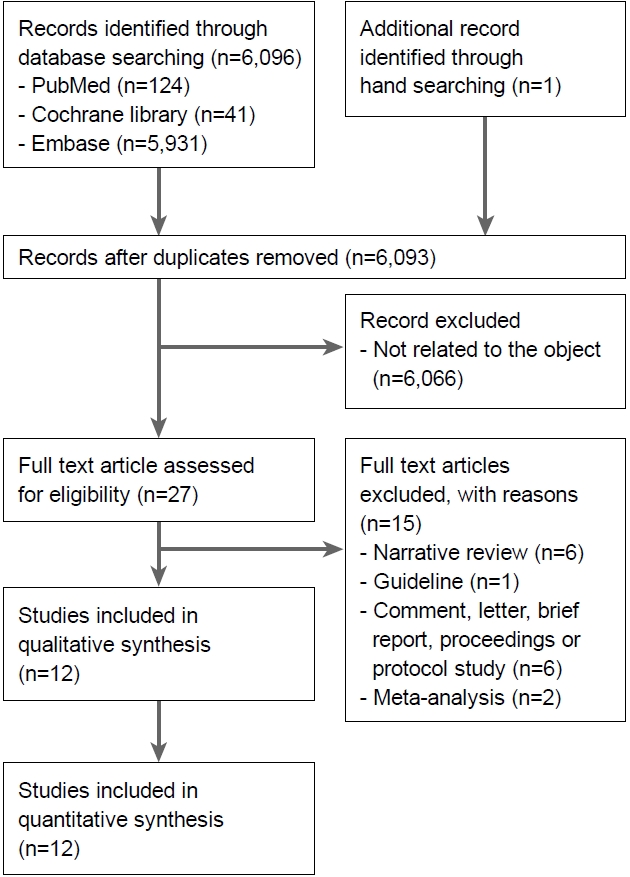
Fig. 1 presents a flow diagram showing how relevant studies were identified. A total of 6,097 articles were identified by searching the three core databases with additional manual searching. Removal of four duplicates resulted in 6,093 articles. During the initial screening through review of titles and abstracts, 6,066 other studies were excluded. Full texts of the remaining 27 studies were then thoroughly reviewed. Among these studies, 15 articles were excluded from the final analysis. The reasons for study exclusion during the final review were as follows: narrative review article (n=6), guideline (n=1), comment, letter, brief report, proceeding, or protocol study (n=6), and meta-analysis (n=2). The remaining 12 studies were included in the final systematic review.
Of the 12 nested, case-control, or cohort studies, a total of 394,582 patients were identified. Seven studies conducted analysis based on cohort from a database [11,12,22-26]. Haenisch et al. [11] analyzed 3,327 persons and identified 431 patients with incident dementia, including 260 patients with AD. Patients receiving PPI medication had significantly higher risk of any dementia (HR, 1.38; 95% CI, 1.04~1.83) and AD (HR, 1.44; 95% CI, 1.01~2.06) compared to those who did not receive PPI. Gomm et al. [12] demonstrated that patients receiving regular PPI medication had significantly increased risk of incident dementia compared to patients not receiving PPI medication (HR, 1.44; 95% CI, 1.36~1.52). Clouston et al. [26] examined whether PPI use was associated with severe cognitive impairment and whether posttraumatic stress disorder explained this association in a cohort of World Trade Center responders. After adjusting for posttraumatic stress disorder, PPI use was significantly associated with severe cognitive impairment (OR, 1.67; 95% CI, 1.054~2.643). Lochhead et al. [24] did not observe a convincing association between PPI use and cognitive function after controlling for histamine-2 receptor antagonists (H2RA) in middle-aged and older women (P=0.34). Gray et al. [23] showed that PPI exposure was not associated with the risk of dementia (P=0.66) or AD (P=0.77). After analyzing data from two large population-based studies of twins in Denmark, Wod et al. [22] found no association between PPI and cognitive decline. Goldstein et al. [25] found that PPIs were not associated with greater risk of dementia or AD. According to their data, not only continuous PPI use, but also intermittent PPI use were associated with lower risk of decline in cognitive function and conversion to mild cognitive impairment or AD. Booker et al. [27] revealed that PPI was associated with decreased risk of developing dementia (OR, 0.96; 95% CI, 0.94~0.99). Herghelegiu et al. [28] showed that prolonged utilization of PPIs resulted in a significant increase of dementia risk after controlling for diabetes and hypertension in midlife (OR, 3.67; 95% CI, 2.23~19.15). Tai et al. [29] reported that cumulative PPI use was significantly associated with dementia (adjusted HR, 1.22; 95% CI, 1.05~1.42). However, Taipale et al. [30] demonstrated that PPI use was not associated with the risk of AD (adjusted OR, 1.03; 95% CI, 1.00~1.05). They also found that neither longer duration of use nor higher dose use was associated with AD. Park et al. [31] performed prescription sequence symmetry analysis to estimate the sequence ratio between PPI use and dementia compared with an active comparator, the use of H2RA. They concluded that the risk of PPIs being associated with dementia might be overestimated. Clinical characteristics of these included studies are shown in Table 1.
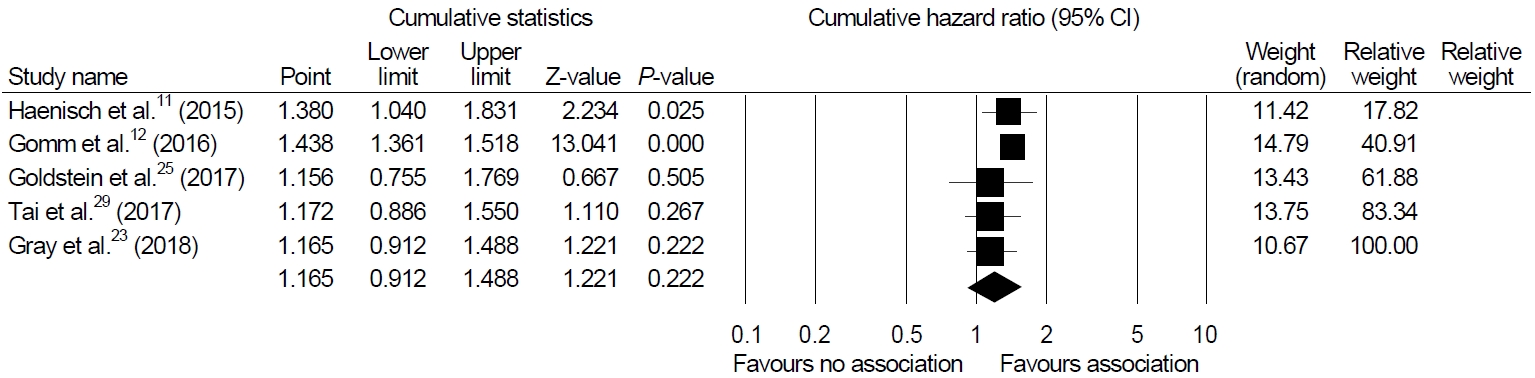
Among the 12 studies included in our systematic review, we were able to perform a meta-analysis using five studies that presented HR as a result [11,12,23,25,29]. The meta-analysis of four cohort studies and one nested case-control study exhibited no association between PPI and dementia (HR, 1.165; 95% CI, 0.912~1.488; P=0.222, I2=91.54%) in a random-effect model analysis (Fig. 2).
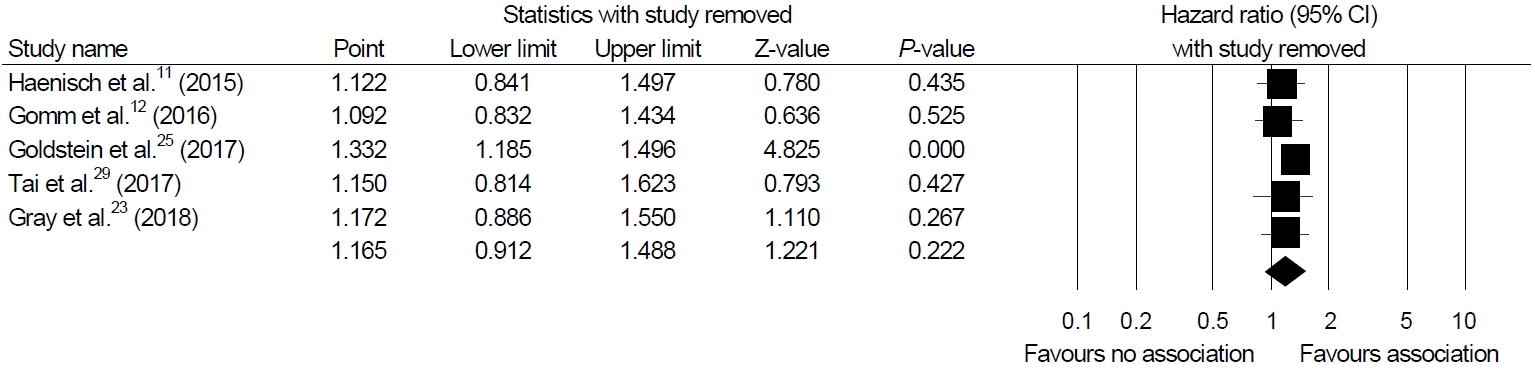
Sensitivity analyses were performed to show the robustness of the results of the main analysis. Cumulative meta-analysis of these enrolled studies in the order of year published showed a significant association in earlier studies but constant non-significant association afterwards (Fig. 3). One-study-removed meta-analysis in the order of year published showed one outlier (study by Goldstein et al. [25], Fig. 4).
The methodological quality of included studies showed similar efficacy. Some studies were reported with a high risk of confounding factors. There were two studies that collected information on PPI exposure by patientsŌĆÖ report (study by Goldstein et al. [25] and Herghelegiu et al. [28]) Detailed methodological quality of enrolled studies is summarized in Fig. 5.
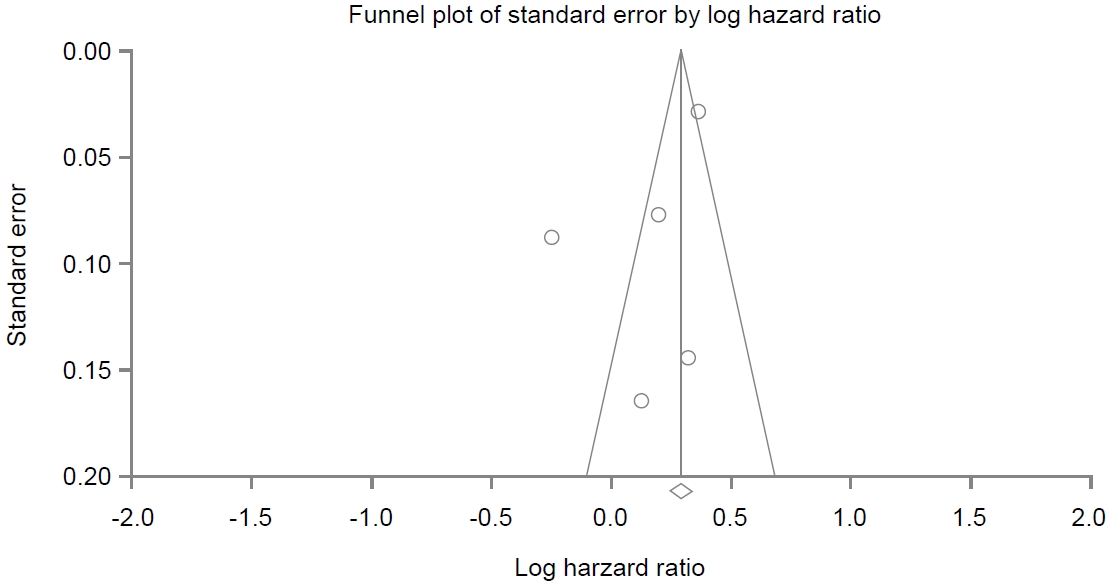
A funnel plot for these included studies in the analysis of association between PPI use and dementia is illustrated in Fig. 6, showing a symmetrical shape. EggerŌĆÖs regression test revealed that the intercept was -3.20 (95% CI, -10.73 to 4.31; P=0.13 [1-tailed] and P=0.27 [2-tailed]). Trim and fill analysis showed that no study was missed or trimmed. Rank correlation test showed that KendallŌĆÖs tau was -0.10 with a continuity correction (P=0.40 [1-tailed] and P=0.80 [2-tailed]). Overall, there was no evidence of publication bias in this meta-analysis.
This meta-analysis of observational studies revealed that PPI use was not associated with an increased risk of dementia. Extensive randomized controlled trials have shown that PPIs are safe with adverse events rates very similar to placebo in the short term [32]. However, long-term use has been associated with various adverse events such as pneumonia, fracture, Clostridium difficile risk, ischemic heart disease, chronic renal failure, and even all-cause mortality [33-38].
Recently, a study of 73,679 participants registered in a German statutory health insurance found that regular PPI users had increased risk of developing dementia, with a HR of 1.44 (95% CI, 1.36~0.152) [12]. Dementia is a clinical condition characterized by progressive cognitive decline that affects oneŌĆÖs ability to live independently. It predominantly affects older adults. In 2016, an estimated 5.4 million Americans have AD. Of these, 5.2 million were those aged 65 years and older [39].
Biologic mechanisms through which PPIs may increase the risk of dementia remain unknown. Some potential mechanisms have been proposed in theory, including the hypothesis that PPI might cause or facilitate the development of beta-amyloid plaques [9]. Accumulation of these materials is a major component of AD [40]. Recent observational studies have reported conflicting results regarding PPI use and development of dementia. Two cohort studies from Germany have reported that the risk of dementia is associated with PPI use [11,12]. However, more recent studies have found no association between PPI use and the development of dementia. A recent systematic review explored the association between PPI use and dementia without reaching a definite conclusion due to methodological issues and conflicting results [13]. That systematic review included 11 studies, including three case reports and one case series [13]. Another meta-analysis reported that PPI users were associated with high risk of dementia [41]. However, this study included few studies, and statistical heterogeneity was high (I2=99%).
We excluded case reports from our review and added eight recently published cohort studies. In the present study, we performed a meta-analysis including a total of 106,451 patients from five studies and found no significant relationship between PPI and dementia (HR, 1.165; 95% CI, 0.912~1.488; P=0.222). In cumulative analysis, the initial two studies were the only studies that showed high magnitude of association between PPI and dementia (Fig. 3) [11,12]. The remaining studies did not find an increased risk of dementia or AD with PPI use.
Our study has several limitations. All studies included in our meta-analysis were primarily observational studies rather than randomized controlled trials. However, randomized controlled trials regarding adverse events of PPI use are difficult to perform. We believe that studies included in our study are currently the best evidence available on this topic. We were able to perform a meta-analysis including five studies that reported the HR of PPI use and dementia. This may have resulted in the high heterogeneity and also implies logical loopholes in obtaining publication bias.
In conclusion, this study indicated that long-term PPI use was not associated with the development of dementia. Considering the small number of studies included in our analysis, our results should be interpreted with caution. Larger studies with longer follow-up duration are needed to further confirm the relationship between PPI use and dementia.
Fig.┬Ā2.
Association between proton pump inhibitor use and cognitive function. The size of each square is proportional to the studyŌĆÖs weight. The diamond is the summary estimate from pooled studies (random-effect model).
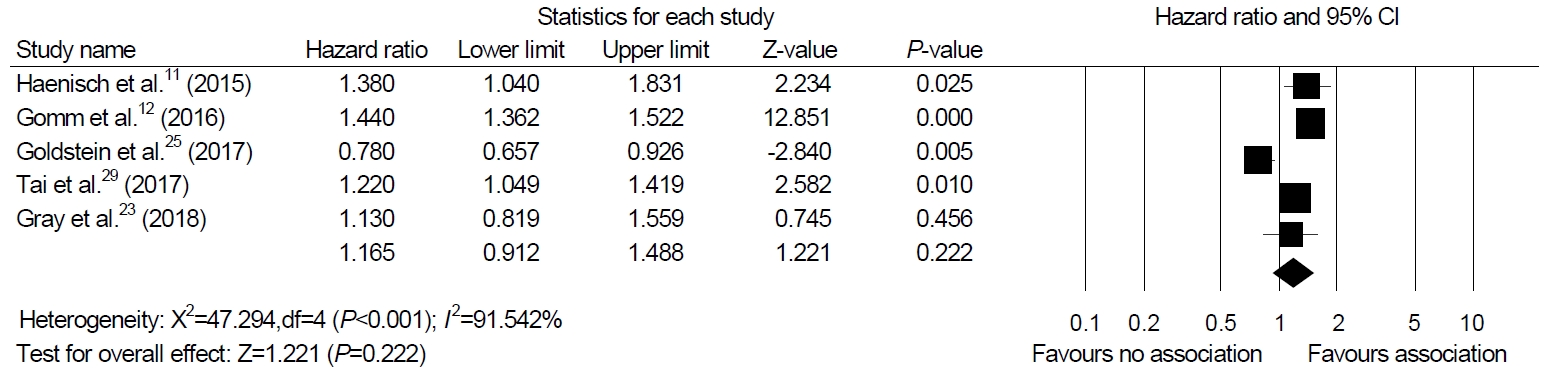
Fig.┬Ā5.
Risk of Bias Assessment tool for non-randomized studies (RoBANS) for the assessment of methodological quality of all enrolled studies.

Fig.┬Ā6.
Funnel plot of enrolled studies regarding proton pump inhibitor use and cognitive function. The line in the center is the natural logarithm of the pooled hazard ratio. The two oblique lines are pseudo 95% confidence limits.
Table┬Ā1.
Clinical Data of Studies That Evaluated the Association between PPI Use and Dementia
| Study | Country | PPI used | Outcome | Study format | Patients and controls | Age (years) |
|---|---|---|---|---|---|---|
| Haenisch et al. [11] (2015) | Germany | Omeprazole, esomeprazole, lansoprazole, pantoprazole, rabeprazole, dexlansoprazole | Dementia: HR, 1.38 (95% CI, 1.04~1.83) | Prospective cohort study | Dementia: PPI users 713, non-users 2,363 | Dementia: PPI users 79.6┬▒3.4, non-users: 79.7┬▒3.6 |
| AD: HR, 1.44 (95% CI, 1.01~2.06) | AD: PPI users 673, non-users 2,238 | AD: PPI users 79.6┬▒3.4, non-users: 79.6┬▒3.6 | ||||
| Booker et al. [27] (2016) | Germany | NA | Dementia: HR, 0.94 (95% CI, 0.90~0.97) | Case control | Dementia 11,956 and controls 11,956 | Dementia: age 80.4┬▒5.3, controls 80.4┬▒5.3 |
| Herghelegiu et al. [28] (2016) | Romania | Omeprazole, pantoprazole, esomeprazole, lansoprazole | Dementia: OR, 3.67 (95% CI, 2.23~19.15) | Cross sectional study | Dementia: PPI users 74, non-users 74 | PPI user: age 76.3┬▒8.7, non-users 74.2┬▒10.3 |
| Gomm et al. [12] (2016) | Germany | Omeprazole, pantoprazole, lansoprazole, esomeprazole, rabeprazole | Dementia: HR, 1.44 (95% CI, 1.36~1.52) | Prospective cohort study from 2004 to 2011 | Dementia: PPI users 2,950, non-users 70,729 | PPI users 83.8┬▒5.4, non-users: 83.0┬▒5.6 |
| CIouston et al. [26] (2017) | USA | NA | Severe cognitive impairment: aOR, 1.67 (95% CI, 1.054~2.643) | Prospective cohort study | No SCI 3696, SCI 83 | No SCI 38.327┬▒8.156, SCI 41.675┬▒8.739 |
| Goldstein et al. [25] (2017) | USA | Omeprazole, esomeprazole, lansoprazole, pantoprazole, rabeprazole, dexlansoprazole | Cognitive impairment: continuous use: HR, 0.78 (95% CI, 0.66~0.93) | Retrospective cohort study | PPI users 2,809, non-users 7,677 | Always PPI users 73.5┬▒8.9, non-users: 72.6┬▒9.4 |
| Intermittent use: HR, 0.84 (95% CI, 0.76~0.93) | ||||||
| Lochhead et al. [24] (2017) | USA | NA | Overall cognition <0.001 | Prospective cohort study | PPI users 2,334, non-users 9,252 | PPI users 61.5┬▒4.6, non-users: 60.9┬▒4.6 |
| Tai et al. [29] (2017) | Taiwan | NA | Dementia: aHR, 1.22 (95% CI, 1.05~1.42) | Nested case-control study | PPI user 7,863, non-user 7,863 | PPI users 55.7┬▒12.4, non-users: 55.3┬▒12.2 |
| Taipale et al. [30] (2017) | Finland | NA | AD: aOR 1.03 (95% CI, 1.00~1.05) | Nested case-control study | PPI user 77,369, non-user 153,206 | Median age 80.8 |
| Gray et al. [23] (2018) | USA | NA | Dementia: 1 year HR, 0.87 (95% CI, 0.65~1.18) | Prospective cohort study | PPI user 402, non-user 3,082 | PPI users 75, non-users 74 |
| 3 year HR, 0.99 (95% CI, 0.75~1.30) | ||||||
| 5 year HR, 1.13 (95% CI 0.82~1.56) | ||||||
| Park et al. [31] (2018) | Korea | Lansoprazole, omeprazole, pantoprazole, rabeprazole, esomeprazole, dexlansoprazole | Dementia: aSR, 1.21 (95% CI, 1.16~1.27) | Population based cohort | PPI user 4,318, non-user 3,024 | NA |
| Wod et al. [22] (2018) | Denmark | NA | Prospective cohort study | PPI user 561, non-user 7,317 | PPI users 56.5, 74.6; non-users 58.0, 75.5 |
Values ard presented as mean┬▒standard deviation or number.
PPI, proton-pump inhibitor; HR, hazard ratio; CI, confidence inerval; AD, alzheimerŌĆÖs disease; NA, not available; OR, odds ratio; aOR, adjusted odds ratio; SCI, severe cognitive impairment; aHR, adjusted hazard ratio; aSR, adjusted sequence ratio.
REFERENCES
1. Hunt RH, Cederberg C, Dent J, et al. Optimizing acid suppression for treatment of acid-related diseases. Dig Dis Sci 1995;40(2 Suppl):24SŌĆō49S.


2. Li Y, Sha W, Nie Y, et al. Effect of intragastric pH on control of peptic ulcer bleeding. J Gastroenterol Hepatol 2000;15:148ŌĆō154.


3. Meineche-Schmidt V. Empiric treatment with high and standard dose of omeprazole in general practice: two-week randomized placebo-controlled trial and 12-month follow-up of health-care consumption. Am J Gastroenterol 2004;99:1050ŌĆō1058.


4. Rabeneck L, Souchek J, Wristers K, et al. A double blind, randomized, placebo-controlled trial of proton pump inhibitor therapy in patients with uninvestigated dyspepsia. Am J Gastroenterol 2002;97:3045ŌĆō3051.


5. Rotman SR, Bishop TF. Proton pump inhibitor use in the U.S. ambulatory setting, 2002-2009. PLoS One 2013;8:e56060.



6. Potteg├źrd A, Broe A, Hallas J, de Muckadell OB, Lassen AT, L├Ėdrup AB. Use of proton-pump inhibitors among adults: a Danish nationwide drug utilization study. Therap Adv Gastroenterol 2016;9:671ŌĆō678.



7. Chen J, Yuan YC, Leontiadis GI, Howden CW. Recent safety concerns with proton pump inhibitors. J Clin Gastroenterol 2012;46:93ŌĆō114.


8. Gill JM, Player MS, Metz DC. Balancing the risks and benefits of proton pump inhibitors. Ann Fam Med 2011;9:200ŌĆō202.



9. Badiola N, Alcalde V, Pujol A, et al. The proton-pump inhibitor lansoprazole enhances amyloid beta production. PLoS One 2013;8:e58837.



10. OŌĆÖLeary F, Allman-Farinelli M, Samman S. Vitamin B12 status, cognitive decline and dementia: a systematic review of prospective cohort studies. Br J Nutr 2012;108:1948ŌĆō1961.


11. Haenisch B, von Holt K, Wiese B, et al. Risk of dementia in elderly patients with the use of proton pump inhibitors. Eur Arch Psychiatry Clin Neurosci 2015;265:419ŌĆō428.



12. Gomm W, von Holt K, Thom├® F, et al. Association of proton pump inhibitors with risk of dementia: a pharmacoepidemiological claims data analysis. JAMA Neurol 2016;73:410ŌĆō416.


13. Batchelor R, Gilmartin JF, Kemp W, Hopper I, Liew D. Dementia, cognitive impairment and proton pump inhibitor therapy: a systematic review. J Gastroenterol Hepatol 2017;32:1426ŌĆō1435.


14. Moher D, Liberati A, Tetzlaff J, Altman DG.; PRISMA Group. Preferred reporting items for systematic reviews and meta-analyses: the PRISMA statement. Ann Intern Med 2009;151:264ŌĆō269; , W64.


15. Kim SY, Park JE, Lee YJ, et al. Testing a tool for assessing the risk of bias for nonrandomized studies showed moderate reliability and promising validity. J Clin Epidemiol 2013;66:408ŌĆō414.


16. Higgins JP, Thompson SG, Deeks JJ, Altman DG. Measuring inconsistency in meta-analyses. BMJ 2003;327:557ŌĆō560.



18. Begg CB, Mazumdar M. Operating characteristics of a rank correlation test for publication bias. Biometrics 1994;50:1088ŌĆō1101.


19. Sterne JA, Egger M. Funnel plots for detecting bias in meta-analysis: guidelines on choice of axis. J Clin Epidemiol 2001;54:1046ŌĆō1055.


20. Duval S, Tweedie R. Trim and fill: a simple funnel-plot-based method of testing and adjusting for publication bias in meta-analysis. Biometrics 2000;56:455ŌĆō463.


21. Leimu R, Koricheva J. Cumulative meta-analysis: a new tool for detection of temporal trends and publication bias in ecology. Proc Biol Sci 2004;271:1961ŌĆō1966.



22. Wod M, Hallas J, Andersen K, Garc├Ła Rodr├Łguez LA, Christensen K, Gaist D. Lack of association between proton pump inhibitor use and cognitive decline. Clin Gastroenterol Hepatol 2018;16:681ŌĆō689.


23. Gray SL, Walker RL, Dublin S, et al. Proton pump inhibitor use and dementia risk: prospective population-based study. J Am Geriatr Soc 2018;66:247ŌĆō253.


24. Lochhead P, Hagan K, Joshi AD, et al. Association between proton pump inhibitor use and cognitive function in women. Gastroenterology 2017;153:971ŌĆō979; e4.


25. Goldstein FC, Steenland K, Zhao L, Wharton W, Levey AI, Hajjar I. Proton pump inhibitors and risk of mild cognitive impairment and dementia. J Am Geriatr Soc 2017;65:1969ŌĆō1974.



26. Clouston SAP, Shapira O, Kotov R, et al. Proton pump inhibitors and the risk of severe cognitive impairment: the role of posttraumatic stress disorder. Alzheimers Dement (N Y) 2017;3:579ŌĆō583.



27. Booker A, Jacob LE, Rapp M, Bohlken J, Kostev K. Risk factors for dementia diagnosis in German primary care practices. Int Psychogeriatr 2016;28:1059ŌĆō1065.


28. Herghelegiu AM, Prada GI, Nacu R. Prolonged use of proton pomp inhibitors and cognitive function in older adults. Farmacia 2016;64:262ŌĆō267.
29. Tai SY, Chien CY, Wu DC, et al. Risk of dementia from proton pump inhibitor use in Asian population: a nationwide cohort study in Taiwan. PLoS One 2017;12:e0171006.



30. Taipale H, Tolppanen AM, Tiihonen M, Tanskanen A, Tiihonen J, Hartikainen S. No association between proton pump inhibitor use and risk of AlzheimerŌĆÖs disease. Am J Gastroenterol 2017;112:1802ŌĆō1808.


31. Park SK, Baek YH, Pratt N, Kalisch Ellett L, Shin JY. The uncertainty of the association between proton pump inhibitor use and the risk of dementia: prescription sequence symmetry analysis using a Korean healthcare database between 2002 and 2013. Drug Saf 2018;41:615ŌĆō624.



33. Xie Y, Bowe B, Li T, Xian H, Yan Y, Al-Aly Z. Risk of death among users of proton pump inhibitors: a longitudinal observational cohort study of United States veterans. BMJ Open 2017;7:e015735.



34. Lazarus B, Chen Y, Wilson FP, et al. Proton pump inhibitor use and the risk of chronic kidney disease. JAMA Inter Med 2016;176:238ŌĆō246.

35. Shah NH, LePendu P, Bauer-Mehren A, et al. Proton pump inhibitor usage and the risk of myocardial infarction in the general population. PLoS One 2015;10:e0124653.



36. Dial S, Delaney JA, Barkun AN, Suissa S. Use of gastric acid-suppressive agents and the risk of community-acquired Clostridium difficile-associated disease. JAMA 2005;294:2989ŌĆō2995.


37. Yang YX, Lewis JD, Epstein S, Metz DC. Long-term proton pump inhibitor therapy and risk of hip fracture. JAMA 2006;296:2947ŌĆō2953.


38. Laheij RJ, Sturkenboom MC, Hassing RJ, Dieleman J, Stricker BH, Jansen JB. Risk of community-acquired pneumonia and use of gastric acid-suppressive drugs. JAMA 2004;292:1955ŌĆō1960.


39. AlzheimerŌĆÖs Association. 2016 AlzheimerŌĆÖs disease facts and figures. Alzheimers Dement 2016;12:459ŌĆō509.



- TOOLS
-
METRICS

-
- 2 Crossref
- 4,991 View
- 122 Download
- Related articles in Korean J Helicobacter Up Gastrointest Res
-
Proton Pump Inhibitors and Gastric Cancer2021 September;21(3)
Acid Secretory Inhibitors and the Risk of Development of Gastric Cancer2019 March;19(1)
Critical Appraisal of Systematic Review/Meta-analysis2015 June;15(2)