Association between Recurrence and Survival Rates According to the Location of Gastric Gastrointestinal Stromal Tumor
Article information
Abstract
Background/Aims
This study aimed to evaluate the clinicopathological parameters of gastric gastrointestinal stromal tumors (GISTs) and to investigate the effect of tumor site on clinical outcomes.
Materials and Methods
Patients treated for a surgically confirmed gastric GIST were retrospectively evaluated between January 2001 and June 2016. The risk level was determined on the basis of the tumor size and number of mitoses. The risk level, recurrence rate, and survival rates were evaluated on the basis of the site of the gastric GISTs.
Results
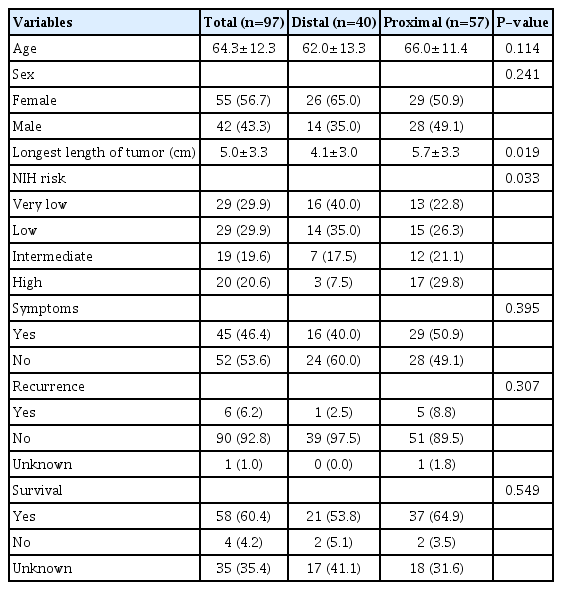
The 97 patients identified included 42 men and 55 women. The median follow-up period was 36 months (range: 12~72 years). Of the 97 patients, 57 (58.7%) and 40 (41.3%) patients had proximally and distally located gastric GISTs, respectively. The high- and low-risk groups had higher proportions of proximally and distally located tumors, respectively (P=0.033). The recurrence rates of proximal and distal GISTs were 8.8%, and 2.5%, respectively (P=0.307). The overall survival rate was not associated with the site of the gastric GISTs (P=0.549).
Conclusions
No relevant associations were found among recurrence, overall survival, and gastric GIST location.
INTRODUCTION
Gastrointestinal stromal tumor (GIST) is a commonly occurring mesenchymal tumor of the gastrointestinal system and consist of interstitial cells of Cajal (ICC) or their stem cells that activate mutations of KIT or PDGFRA protooncogenes that are involved in oncogenicity of GISTs [1,2]. GISTs occur mainly in adults and the median age is 55 to 60 years [3]. About 80% of GISTs are expressed as mesenchymal tumors and account for less than 3% of all gastrointestinal malignancies [4-7]. The most common sites for GIST are stomach (70%), small intestine (20~30%), colon (5~10%) and esophagus (5%) [8]. Although the Swedish population-based study [3] and the pathological study of the Armed Forced Institute of Pathology [9] have been reported, there is limited research on the relevance and outcomes of gastric GISTs risk according to the location of gastric GISTs. Previous studies have concentrated on the overall GIST of the whole. There were several clinicopathologic studies focused on gastric GISTs, but they were performed in small groups [10-12]. The prognosis of patients with GISTs might be related to the anatomical site of the tumor [13]. The incidence of gastric GISTs is known to be generally low in lower third of the stomach [10-12]. However, these studies did not elucidate the reasons for the incidence rates based on the location of gastric GISTs. In order to determine whether the site of GISTs in the stomach affects a specific prognosis, further analysis of the clinical outcome according to the GIST location in the stomach is necessary. This study was designed to predict the clinical outcome based on the site of gastric GISTs retrospectively in multicenter and to identify clinicopathologic factors and survival rates of patients with primary gastric GISTs.
MATERIALS AND METHODS
1. Patients
This multicenter, retrospective study was performed at three Catholic University Hospitals in Korea. Between January 2001 and June 2016, the medical records of 97 GIST patients who were confirmed by surgery were reviewed. Medical records were reviewed for clinical parameters, including gender, age, tumor site, symptoms, surgery, chemotherapy, and pathological characteristics. The patients were subdivided into three groups. The location of gastric GISTs was divided anatomically into upper third, middle third and lower third portion. The upper third and middle third parts were then combined to form the proximal portion and the lower third to the distal portion.
2. Pathology
Immunohistochemical staining analysis was performed to identify immunophenotypes. Immunohistochemistry processing was performed via antibodies against CD117 (c-kit), S-100 protein and CD-34 and SMA-alpha. Almost all patients were confirmed as gastric GISTs by immunohistochemical staining for CD117 (c-kit), CD34, or both. The definition of pathological diagnosis in GISTs, which is confirmed by cell morphology and immunohistochemical staining. The risk was classified as very low, low, intermediate, and high according to National Institute of Health (NIH) risk-group stratification system [1]. Pathological parameters were evaluated, including mitotic count, tumor size, and cellularity. The tumor size was measured as the longest length. The mitotic counts were evaluated on 50 high power fields. Eophagogastroduodenoscopy and CT were done as a tool for preoperative examination.
3. Prognosis analysis
The survival period was evaluated from the day of surgery to death, related to the disease or not. In addition, this period was calculated based on whether it was related to the disease and whether it was censored for the latest follow-up observations. Follow-up studies were performed as follows: chest X-ray, esophagogastroduodenoscopy, abdominal CT scans, chest CT scans, and/or PET scans. Recurrence was assessed according to the medical records. Tumor sites of the stomach and risk group were used for survival analysis.
4. Statistical analysis
Categorical data were presented as numbers (%). They were compared using the chi-square test. Continuous variables were represented as the mean±standard deviation, and were compared between groups using the Student’s t-test. Survival curves were constructed using a Kaplan-Meier survival analysis with comparisons between the curves based on a log-rank χ2 statistic. A P-value of less than 0.05 was considered to be statistically significant. Statistical analysis was performed using SPSS® Statistics 21.0 (IBM, Armonk, NY, USA).
RESULTS
1. Clinical characteristics
Ninety seven patients were diagnosed with primary gastric GISTs. There were 42 males (43.3%) and 55 females (56.7%), with a mean age of 64.3±12.3 (SD) years. While 45 (46,4%) patients had symptoms such as abdominal pain, dyspepsia, fatigue, palpable mass, gastrointestinal bleeding and anemia, the rest of them were asymptomatic, incidentally found during an annual screening examination or evaluation of other disease.
2. Histopathologic features
Average longest length of tumor is 5.0±3.3 (SD) cm. Immunohistochemical staining demonstrated that 82 tumors (86.3%) were positive for CD117 and 83 (85.6%) were positive for CD34. The patients was classified by risk group according to the NIH criteria. Based on the NIH criteria, 29.9% of patients were distributed in the very low risk group, 29.9% were in the low risk group, 19.6% were intermediate risk, and 20.6% were high risk. Four risks were divided into low risk and high risk. The low-risk group had low or very low risk patients and the high-risk group included patients with an intermediate or high risk.
3. Tumor site and outcome
Of the 97 tumors, 57 (58.3%), and 40 (41.7%) were located in the proximal and the distal part of the stomach, respectively. The median follow-up period was 36 months (range: 12~72). Table 1 compared the characteristics in distal and proximal portions of the gastric GISTs, which indicated that proximally located tumors had much more cases of high risk group than distal tumors (P =0.033). The average length of tumor was statistically significantly longer in the proximal part of the tumor than in the distal part (P =0.019). The recurrence rate of proximally located GISTs was 8.8%, and for distally located GISTs, it was 2.5% (P =0.307). In addition, the overall survival rate did not correlate with the site of the gastric GISTs (P >0.999).
4. Prognostic evaluation
The patients were classified into two groups based on risk of gastric GISTs. There was no significant difference in overall survival between the two groups of patients in Kaplan-Meier curve (P =0.618) (Fig. 1). The survival curve was also analyzed according to the location of GISTs in the stomach. There was no statistically significant difference between the proximal part (upper third+middle third part of the stomach) and the distal part (lower third part of the stomach) of the gastric GISTs (P =0.237) (Fig. 2).

Kaplan-Meier curves of survival probability rates according to two risk groups of gastric gastrointestina stromal tumors. No signi ficant differences are observed in overall survival between the two groups of patients (P=0.618).
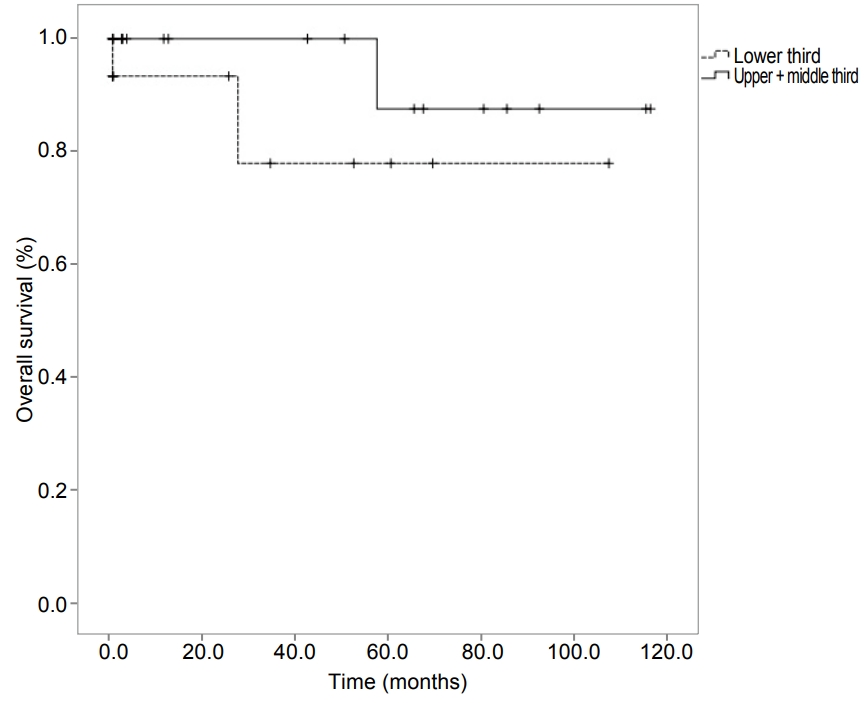
Kaplan-Meier curves of survival probability rates according to the two sites of gastric gastrointestina stromal tumors. No statistically significant difference is observed between the proximally (upper third+middle third part of the stomach) and distally located gastric gastrointestinal stromal tumors (lower third part of the stomach; P=0.237).
DISCUSSION
In this study, we assessed the recurrence and survival rate according to the location of gastric GISTs, however, we did not demonstrate the usefulness of the location of gastric GISTs in assessing the risk and prognosis. We found the large size of the GIST tumors in the proximal portion of the stomach than in the distal stomach.
As prognostic factors of GISTs, the following facts were revealed: age, sex, tumor site, tumor size, incomplete resection, mitotic count, KIT mutations, mucosal involvement, operative variables, proliferative index, and epithelioid subtype. Among these, mitotic activity, tumor size, risk stratification and complete surgical resection were essential prognostic parameters of primary GISTs, which was confirmed in several studies [14,15]. Based on the NIH risk criteria for tumor sizes and mitotic counts, the proximal portion of the stomach may be at higher risk because the size of the tumor is larger in the proximal portion of the stomach. The number of mitotic counts is known to be the most important parameter to determine the malignant tumor in the prognosis of GISTs and high mitotic activity is known to be a sign of active growth and tumor malignancy. Therefore, the higher the mitotic count, the more likely it is to develop malignancy tumor, which is likely to affect the prognosis of GIST patients. If there would be evidence of other malignant features despite of low mitotic count, a malignant GISTs should still be diagnosed. Tumor size is only marginally correlated with malignant tumors and is less important than histopathological factors [16]. In a study analyzed 1,756 cases of gastric GISTs, it was found that the location of gastric GISTs in the fundus or gastroesophageal junctions was unfavorable factors, whereas it was favorable factors in the antrum [17]. One possibility is that the changes in the proliferative properties of ICC cells and stromal smooth muscle stem cells may differ depending on the anatomical location of the stomach.
There are several limitations to our study. We could not evaluate the risk by subdividing the mitotic count and tumor size according to the site of gastric GISTs. The patients who failed to follow up after surgery was relatively high, and mutation analysis and pathologic analysis of GISTs were not performed in this study.
In conclusion, there is no relevance between the recurrence rate, the overall survival rate, and the site of the gastric GISTs in our study. The proximal GISTs may be an unfavorable factor, it seems that the proximal portion of gastric GISTs is larger than the distal portion.
Notes
No potential conflict of interest relevant to this article was reported.