Changes in Helicobacter pylori Immunoglobulin G Levels and Gastric Mucosal Atrophy after Successful Eradication of Helicobacter pylori
Article information
Abstract
Background/Aims
Limited information is available about the relationship between Helicobacter pylori (H. pylori) immunoglobulin (Ig) G and serum pepsinogen (pepsinogen [PG], a marker of gastric mucosal atrophy) concentrations after H. pylori eradication.
Materials and Methods
Eligible patients who underwent endoscopic submucosal dissection (ESD) for early gastric cancer from August 2007 to March 2013 in a tertiary-referral center, and whose serum H. pylori IgG and PG concentrations were measured at the time of performing ESD and one year post-ESD, were selected. Successful H. pylori eradication was achieved after ESD in all the patients. According to the decrease in serum H. pylori IgG concentration after bacterial eradication, the patients were categorized as group 1 (IgG concentration decreased by <50%), and group 2 (IgG concentration decreased by ≥50%).
Results
Of the 106 patients, 25 (23.6%) were classified into group 1 and 81 (76.4%) into group 2. One year after H. pylori eradication, the serum PG II concentration was significantly decreased in group 2 (12.46±8.18 vs. 8.28±6.11, P=0.024). Although the serum PG I/II ratio of group 2 was higher than that of group 1 (8.32±4.52 ng/mL vs. 6.39±4.04 ng/mL), the difference was not significant (P=0.058). One year after successful eradication, elevated serum PG I/II ratio was observed in 21 patients (84%) in group 1 and in 77 patients (95.1%) in group 2 (P=0.087). The mean serum PG I/II ratio was also elevated in both groups. Serum PG II concentration was significantly decreased in group 2.
Conclusions
A notable decrease in the concentration of H. pylori IgG antibody after bacterial eradication might reflect gastric mucosal atrophy. However, our study showed no statistically significant difference.
INTRODUCTION
Helicobacter pylori (H. pylori) infection causes gastric mucosal atrophy, intestinal metaplasia, and gastric cancer [1]. For the diagnosis of this bacterial infection, invasive methods such as endoscopic biopsy or noninvasive methods such as obtaining specimens other than gastric mucosal tissue were generally performed [2-3]. Several invasive and noninvasive methods are used to monitor the H. pylori status after eradication therapy [4]. Although H. pylori serology is noninvasive, fast, and economical, it is not used for the confirmation of H. pylori eradication because the natural history and biological half-life of H. pylori immunoglobulin (Ig) G are uncertain, and this method could not discriminate between current and past exposure to H. pylori infection [5]. Thus, H. pylori IgG serology could be used for the determination of reinfection of H. pylori, but not for the confirmation of H. pylori eradication [6]. However, even after a successful H. pylori eradication, the antibody concentrations remain high for a substantial period [7-8].
To improve the diagnostic accuracy of serum H. pylori IgG serology performed after treatment, serum pepsinogen (PG) and gastrin-17 were evaluated for their potential as markers for successful bacterial eradication [9-11]. Generally, serum PGs are categorized into two types: PG I, which is mainly produced in the chief cells and mucous neck cells of the corpus, and PG II, which is produced from both those cells and the cardiac, pyloric, Brunner gland cells [12]. Several studies have suggested that the concentration of serum PG is regarded as an objective and reliable parameter in estimation of the extent in chronic atrophic gastritis, and a useful indicator for gastric cancer [12-14]. However, there are few studies about the changes in serum H. pylori IgG antibodies and their effect on the change in gastric mucosal atrophy after a successful H. pylori eradication. Furthermore, the clinical significance between the concentration changes of H. pylori IgG after H. pylori eradication and gastric mucosal atrophy remains uncertain. In this study, we evaluated the relationship between the changes in H. pylori IgG concentration and the status of gastric mucosal atrophy based on the serum PG concentration.
MATERIALS AND METHODS
1. Patient selection
This single-center cohort study included all eligible patients who received endoscopic submucosal dissection for early gastric cancer at Kyungpook National University Hospital, Daegu, Korea, from August 2007 to March 2013. Patients with proven H. pylori infection and who underwent measurements of serum H. pylori IgG antibody and PG concentrations at the time of endoscopic resection and at follow-up evaluations after successful H. pylori eradication were finally enrolled into this retrospective study. Patients with previous H. pylori eradication, eradication failure, no eradication therapy after endoscopic resection, H. pylori reinfection or recrudescence after eradication during the follow-up period, a history of gastrectomy, loss to follow-up after eradication within one year, and continuous drug administration that can affect serum PG concentrations (proton pump inhibitors [PPIs]) were excluded. This study was approved by the University Hospital Kyungpook Trust (KNUMC_13-1059).
2. Determination and eradication of H. pylori
A presence H. pylori infection was confirmed when at least two tests provided evidence of H. pylori infection, including histological test, rapid urease test (CLOtest; Delta West, Bentley, Australia), and serum H. pylori IgG antibody test (Diesse, Monteriggioni, Italy). All patients with H. pylori infection at the time of endoscopic resection were recommended to receive the conventional PPI-based triple therapy (standard dose of PPI twice daily [bid], clarithromycin 500 mg bid, and amoxicillin 1 g bid, all for 1 week) for H. pylori eradication. Patients who failed to the first eradication treatment, as revealed by a 13C-urea breath test (Otsuka Pharmaceutical Co. Ltd., Tokyo, Japan), were recommended to received the second-line eradication therapy with a bismuth-containing quadruple regimen (standard dose of PPI bid, tripotassium dicitrate bismuthate 300 mg four times daily, metronidazole 500 mg three times daily, and tetracycline 500 mg four times daily, all for 1 week). Successful eradication was determined using the 13C-urea breath test or an endoscopic rapid urease test. Patients were followed up regularly with endoscopic biopsy and rapid urease test for the evaluation of H. pylori reinfection or recrudescence.
3. Serological analyses at the time of endoscopic submucosal dissection
In the routine laboratory tests before endoscopic submucosal dissection (ESD), sera were obtained from fasting blood samples. The patients’ serum samples were centrifuged and stored at -20℃ before analysis. The serum concentrations of anti-H. pylori IgG antibodies were measured using Vidas H. pylori IgG (BioMérieux, Marcy-l’Etoile, France). This assay, which utilizes the enzyme immunoassay sandwich method, is combined with a final fluorescent detection, and thus is described as an enzyme-linked fluorescent assay. The result was considered positive when the test value was ≥1.00 and negative when the test value was <0.75. Samples with values between these cutoff concentrations were considered equivocal. The manufacturer’s guidance in sensitivity and specificity for the Vidas H. pylori IgG test are 98.1% (95% confidence interval [CI]: 93.1~99.8%) and 90.82% (95% CI: 83.3~95.7%), respectively [15]. Serum PG concentrations were also analyzed by immediately centrifuging the samples at 4℃ and storing the serum at -20℃. And then, the serum PG I and II concentrations were measured using a chemiluminescent immunoassay with the ARCHITECT analyzer (Wako Pure Chemical Industry, Osaka, Japan).
4. Surveillance
The endoscopic surveillance (three months, nine months, and annually after endoscopic resection) was provided for all patients. During this, biopsy and the rapid urease test were performed for confirmation of H. pylori infection. The last surveillance was defined as the last time at which both endoscopic surveillance and serum H. pylori IgG and PG concentration tests were conducted after endoscopic resection. At the visits for endoscopic surveillance, blood samples were collected after 12 hours of fasting. Then, serum H. pylori IgG, PG I, PG II, and the PG I/II ratio were determined after checking whether the patients were using PPIs through a survey [16].
5. Statistical analysis
The enrolled patients were divided into two groups according to the change in serum H. pylori IgG concentration. Group 1 was defined as patients with a <50% decrease in H. pylori IgG after eradication, and group 2 as those with a ≥50% decrease after eradication. In each group, H. pylori IgG antibodies, PG I, PG II, and PG I/II ratio were measured before and one year after successful H. pylori eradication, and then followed up. All continuous variables were expressed as mean±standard deviation. All categorical variables were expressed as number (percentage). The groups were analyzed in terms of continuous and categorical variables, by using Pearson’s chi-square test and unpaired t-test (for comparisons between two groups) or analysis of variance with post-hoc Tukey honest significant difference, respectively. To determine whether there was a significant change from baseline in serum H. pylori IgG antibodies, PG concentrations, and the serum PG I/II ratio after eradication of H. pylori infection, paired t-test was used if normality was satisfied. A commercial statistical software (SPSS for Windows version 21.0; SPSS Inc., Chicago, IL, USA) was used for data analysis. Two-tailed P-values of <0.05 were considered statistically significant.
RESULTS
1. Baseline characteristics of the cohort
Of 402 patients with H. pylori infection at the time of ESD, 106 patients were finally enrolled in this study, in a consecutive manner (Fig. 1). Of them, 88 patients (83.0%) had a successful eradication with the first eradication regimen, whereas 18 patients (17.0%) underwent second-line treatment. The baseline characteristics of the enrolled patients are shown in Table 1.
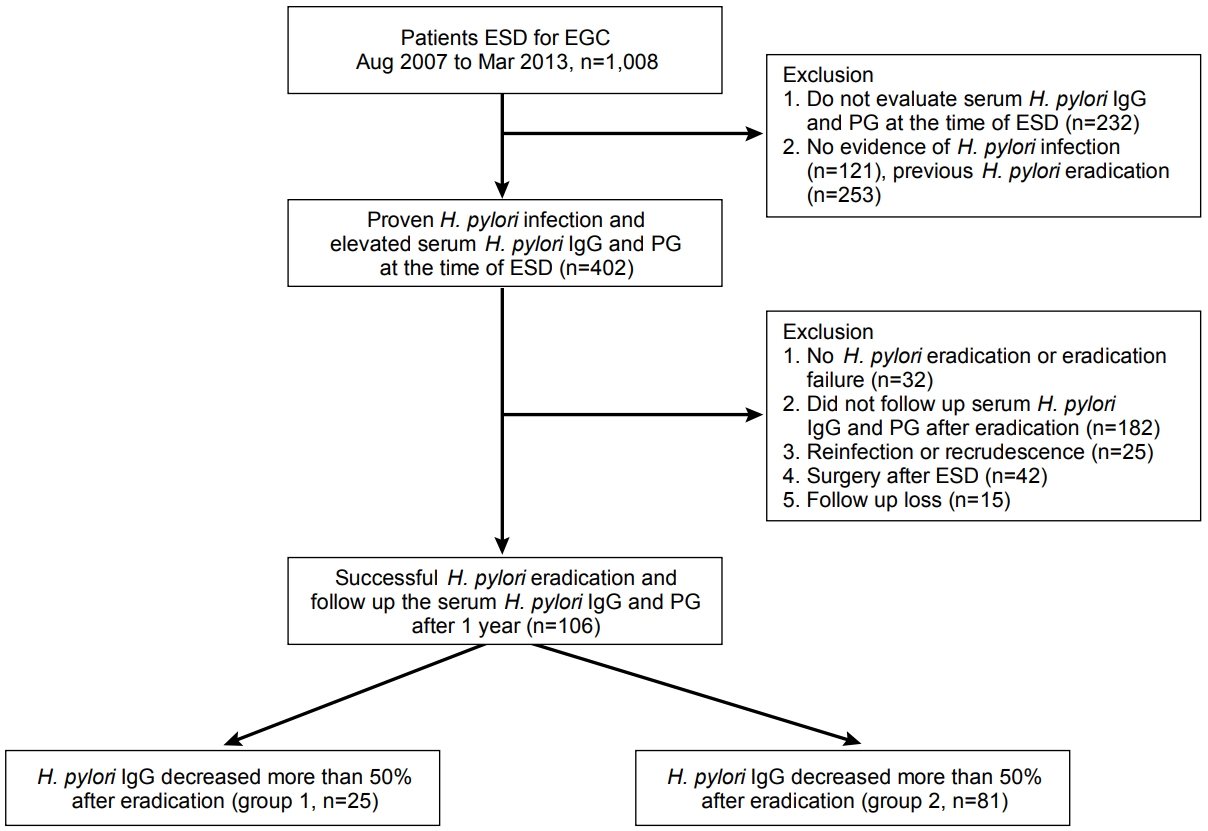
Schematic flow of this study. ESD, endoscopic submucosal dissection; EGC, early gastric cancer; H. pylori, Helicobacter pylori; IgG, immunoglobulin G; PG, pepsinogen.
2. Changes in serum H. pylori IgG antibodies and PG concentrations after H. pylori eradication
All patients underwent measurements of H. pylori IgG antibodies at one year after successful eradication. Endoscopic biopsy and rapid urease test showed no evidence of H. pylori infection in these patients at 1 year after eradication. After successful H. pylori eradication, the serum H. pylori IgG antibody concentrations were decreased in 100 patients and elevated in six patients, compared with the values at the time of ESD. The mean serum H. pylori IgG antibody concentrations were significantly decreased at 1 year after successful H. pylori eradication (P<0.001) (Fig. 2A). The serum PG I and II concentrations were significantly decreased at 1 year after successful H. pylori eradication (P<0.001) (Fig. 2B, C), and the serum PG I/II ratio was significantly increased after successful H. pylori eradication (P<0.001) (Fig. 2D). Finally, 36 (35.3%), 9 (4.9%), and 61 (59.8%) patients showed negative, indeterminate, and positive H. pylori IgG serology results after successful eradication. The serum PG I/II ratio was not statistically different in each group (7.50±4.10 in the negative group, 7.49±4.08 in the indeterminate group, and 8.12±4.76 in positive group, P =0.778).

Changes in serum H. pylori IgG antibody and PG concentrations after 1 year of successful H. pylori eradication in the whole cohort. Changes in (A) serum H. pylori IgG antibody concentrations, (B) PG I concentrations, (C) PG II concentrations, and (D) PG I/II ratios, before and 1 year after eradication, in the whole patient cohort (n=106). H. pylori, Helicobacter pylori; IgG, immunoglobulin G; PG, pepsinogen.
3. Changes in serum PG concentration according to reduction in serum H. pylori IgG antibodies after successful H. pylori eradication
In 25 patients (23.6%), the H. pylori IgG concentration was decreased by <50% compared with before eradication (group 1). On the other hand, in 81 patients (76.4%), the H. pylori IgG concentration decreased by ≥50% (group 2). The patients’ characteristics are shown in Table 2. The mean serum H. pylori IgG antibody and PG concentrations before eradication did not show a statistical difference between two groups. The serum PG I and II concentrations were decreased and the PG I/II ratio was elevated compared with the baseline serum PG concentration. However, serum PG II was significantly decreased in group 2 at 1 year after H. pylori eradication (P =0.024) (Table 2). Although the serum PG I/II ratio was more elevated in group 2 than in group 1 (8.32±4.52 vs. 6.39±4.04), there was no significant statistical difference (P =0.058) (Table 2). In 21 patients (84.0%) in group 1 and 77 patients (95.1%) in group 2, elevated serum PG I/II ratio was observed at 1 year after successful H. pylori eradication (P =0.087). Moreover, the mean serum PG I/II ratio was also elevated in both groups (Fig. 3).

Baseline Characteristics and Change in Serum Biomarkers Based on the Change in H. pylori IgG Levels after 1 Year of Successful H. pylori Eradication
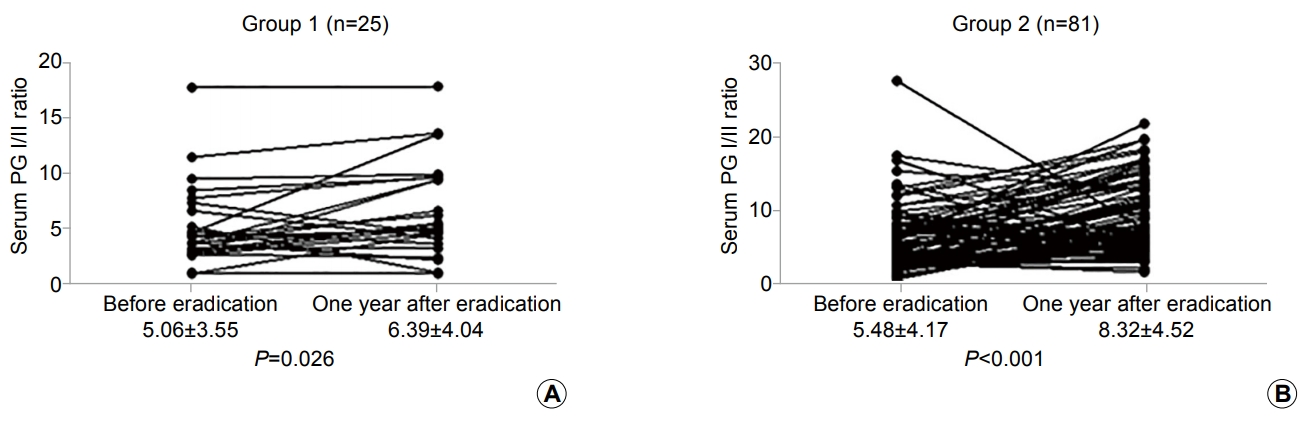
Changes in gastric mucosal atrophy reflected by the serum pepsinogen (PG) I/II ratio based on the changes in serum Helicobacter pylori immunoglobulin (Ig) G antibody concentration after 1 year of successful eradication. The changes in the serum PG I/II ratio before and 1 year after eradication in patients with a <50% decrease (group 1, n=25) (A) and in those with ≥50% decrease (group 2, n=81) (B) in H. pylori IgG levels after eradication are shown.
4. Changes in serum H. pylori IgG antibody and PG concentrations after successful H. pylori eradication during long-term follow-up
Of the patients, 13 (52.0%) in group 1 and 55 (67.9%) in group 2 were followed up for 2 or more years after successful H. pylori eradication (mean 28.5±21.1 months; mean 29.2±20.9 months in group 1 and 25.9±22.9 months in group 2, P =0.381). The serum H. pylori IgG antibodies were significantly decreased during the follow-up period in both groups (Fig. 4A, B). Of these patients, seven (10.3%) showed a <50% decrease in H. pylori IgG antibodies compared with before eradication and 61 (89.7%) showed a ≥50% decrease compared with before eradication, in the final assessment. However, the serum PG I/II ratio was not significantly changed during the long-term follow-up in both groups (Fig. 4C, D).

Long-term follow-up measurements of serum H. pylori IgG antibody and PG I/II ratio according to the changes in serum H. pylori IgG levels. Results are shown for group 1 patients (n=13) with a <50% decrease and group 2 patients (n=55) with a ≥50% decrease in H. pylori IgG levels after 1 year of eradication with follow up for >2 years. (A, B) Change in serum H. pylori IgG levels at 1 year after eradication and at long-term follow up, respectively. (C, D) Change in PG I/II ratio at 1 year after eradication and at long-term follow up, respectively. H. pylori, Helicobacter pylori; IgG, immunoglobulin G; PG, pepsinogen.
DISCUSSION
In this study, we investigated whether the change in H. pylori IgG levels after successful H. pylori eradication is related to the serum PG concentrations. We observed that serum PG II was significantly decreased at 1 year after eradication in the group with a ≥50% decrease in H. pylori IgG antibodies after successful eradication (group 2) compared with the group with a <50% decrease in H. pylori IgG antibodies after eradication (group 1) (8.28±6.11 vs. 12.46±8.18, P =0.024). Although there was no significant statistical difference, a greater decrease in H. pylori IgG antibodies after eradication was associated with a higher increase in the serum PG I/II ratio in our study (6.39±4.04 in group 1 vs. 8.32±4.52 in group 2). During the follow-up at 2 years or more, although H. pylori IgG antibodies decreased in most patients, the serum PG I/II ratio did not show significant changes after successful H. pylori eradication.
Many studies have reported that H. pylori eradication contributes to improving gastric mucosal atrophy [17-19]. Previously, we also reported that the H. pylori eradication ameliorates the risk of development of metachronous gastric neoplasia in patients who underwent endoscopic resection, and it was associated with elevated serum PG I/II ratios in the follow-up, suggesting that the eradication improved the gastric mucosal status [16]. In addition, several studies have attempted to show whether changes in serum PG was related to successful bacterial eradication [9,10,13,20]. Generally, elevated serum PG I and PG II concentrations aggravate the H. pylori-associated non-atrophic superficial gastritis, and these elevated PG concentrations were reported to decrease at one year after the eradication of H. pylori [20]. This decrease originates from the decreased severity of H. pylori-associated gastritis and from the clearing of H. pylori lipopolysaccharide [21-23]. Although H. pylori eradication reduced the concentrations of both PG I and PG II, a greater decrease as observed in PG II than in PG I [9,20,24].
On the basis of our and previous results, serum PG II concentrations could be a reliable parameter during the surveillance period of patients after treatment. The serum PG II concentration increases more than the PG I concentration in H pylori-infected patients, and serum PG II correlates proportionally to the severity of H. pylori-induced gastric inflammation in the antrum and corpus better than serum PG I [25]. Furthermore, H. pylori-induced gastritis is localized mainly in the antrum, and this could affect the concentration of serum PG II more than PG I [25].
Thus, H. pylori eradication improves the gastric mucosal status. However, the correlation between the change in H. pylori serum level after H. pylori eradication and the different stages of gastric mucosal atrophy has not been well investigated. In this study, H. pylori IgG was measured before and after eradication, and the patients were divided according to the degree of reduction in H. pylori IgG after eradication. Although there was no statistical significance, both the PG I concentration and the PG I/II ratio after eradication were higher in group 2. In particular, the difference in PG I/II ratio between before and after eradication was larger. Thus, the degree of H. pylori IgG reduction indicates effective eradication. In this group, the improvement of gastric cell secretion and the reversibility of atrophic gastritis can be predicted by the increase in the PG I/II ratio. A previous Japanese study reported that similar PG I/II concentrations were observed at 2 months after eradication, and comparable concentrations were observed at 12 and 24 months after treatment. The PG I/II ratio is a widely accepted indicator for gastric atrophy, and an increase in this ratio is also considered as an result of the successful H. pylori eradication [26]. Another study also reported that the percentage changes in serum PG I/II ratios revealed the high relation to the outcome of eradication therapy for H. pylori [11]. Taken together, H. pylori eradication contributes to the improvement of gastric cell secretion and the reversibility of atrophic gastritis, and the reduction ratio of H. pylori IgG antibodies might be related to the improvement of gastric mucosal atrophy. However, there was no significant difference during the long-term follow-up. Most important, the sample size was decreased during the follow-up, and other factors such as age, sex, height, body weight, body surface area, smoking, and drinking habits could be related to the PG I and PG II concentrations during the long-term follow-up [13,27,28].
In the present study, we could not show a statistically significant improvement of gastric mucosal atrophy based on the reduction ratio of H. pylori IgG antibodies after H. pylori eradication. This study has some other limitations. Our study was a retrospective study with a small cohort, and only 26.4% of the patients (106 of 402) could be followed up for the evaluation of both serum H. pylori IgG antibody and PG concentrations after successful eradication. The National Cancer Screening Program in Korea offers either endoscopic surveillance or serum PG measurements after endoscopic resection for gastric neoplasms. Thus, we could not follow up all patients for serum PG concentrations after endoscopic resection. Second, we could not evaluate the status of gastric mucosa by using of biopsy. This might be the reason for the difficulties in validating the correlation between serum PG concentrations and gastric mucosal atrophy. However, several previous studies have validated this correlation [29,30]. Further studies are needed to target more patients in the future.
In conclusion, a higher decrease in H. pylori IgG antibody concentration was related to a higher decrease in serum PG II at 1 year after H. pylori eradication. However, gastric mucosal atrophy assessed using the serum PG I/II ratio did not show statistical significance in the group with a higher decrease in H. pylori IgG antibody concentration at 1 year after H. pylori eradication.
Notes
No potential conflict of interest relevant to this article was reported.

