 |
 |
- Search
| Korean J Helicobacter Up Gastrointest Res > Volume 22(2); 2022 > Article |
|
Abstract
Background/Aims
Materials and Methods
Results
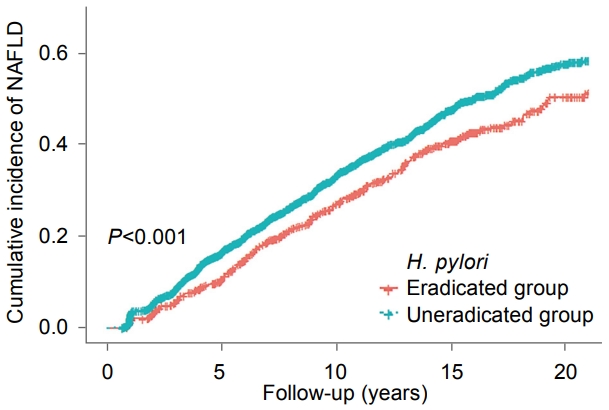
Fig.┬Ā2.
Table┬Ā1.
Values are expressed as means┬▒standard deviation, number, or median (interquartile range).
H. pylori, Helicobacter pylori; BMI, body mass index; BP, blood pressure; FBG, fasting blood glucose; LDL-C, low-density lipoprotein cholesterol; HDL-C, high-density lipoprotein cholesterol; ALT, alanine aminotransferase; GGT, gamma-glutamyltransferase; hsCRP, high-sensitivity C-reactive protein.
Table┬Ā2.
| Model 1a | Model 2b | Model 3c | |
|---|---|---|---|
| H. pylori-eradicated group | 1.00 (reference) | 1.00 (reference) | 1.00 (reference) |
| Uneradicated group | 1.31 (1.14~1.51) | 1.36 (1.19~1.56) | 1.36 (1.18~1.56) |
Table┬Ā3.
NAFLD, non-alcoholic fatty liver disease; HR, hazard ratio; CI, confidence interval; BMI, body mass index; BP, blood pressure; FBG, fasting blood glucose; LDL-C, low-density lipoprotein cholesterol; HDL-C, high-density lipoprotein cholesterol; ALT, alanine aminotransferase; GGT, gamma-glutamyltransferase; hsCRP, high-sensitivity C-reactive protein; H. pylori, Helicobacter pylori.
Table┬Ā4.
| Subgroup | HR (95% CI) | P for interaction |
|---|---|---|
| Age | 0.467 | |
| ŌĆā<50 years | 1.32 (1.09~1.59) | |
| ŌĆāŌēź50 years | 1.44 (1.17~1.78) | |
| Sex | 0.484 | |
| ŌĆāFemale | 1.41 (1.14~1.75) | |
| ŌĆāMale | 1.31 (1.09~1.57) | |
| Obesity (BMI) | 0.968 | |
| ŌĆā<25 | 1.34 (1.02~1.75) | |
| ŌĆāŌēź25 | 1.31 (1.12~1.54) | |
| Current smoking | 0.978 | |
| ŌĆāNo | 1.36 (1.15~1.61) | |
| ŌĆāYes | 1.39 (1.07~1.79) | |
| Modest alcohol intake | 0.753 | |
| ŌĆāNo | 1.34 (1.13~1.58) | |
| ŌĆāYes | 1.41 (1.08~1.82) | 0.347 |
| Metabolic abnormalitiesa | ||
| ŌĆāNo | 1.46 (1.17~1.83) | |
| ŌĆāYes | 1.25 (1.05~1.49) |
Estimated from cox proportional hazard models. Multivariable model was adjusted for age, sex, BMI, smoking status, alcohol intake, systolic blood pressure, fasting blood glucose, triglycerides, low-density lipoprotein cholesterol, high-density lipoprotein cholesterol, alanine aminotransferase, gamma-glutamyltransferase, and high-sensitivity C-reactive protein.
NAFLD, non-alcoholic fatty liver disease; H. pylori, Helicobacter pylori; HR, hazard ratio; CI, confidence interval; BMI, body mass index.
a Metabolic abnormalities had any of the following metabolic abnormal parameters (i) high serum triglycerides, defined as triglycerides Ōēź150 mg/dL; (ii) low high-density lipoprotein-cholesterol (HDL-C) level, defined as HDL-C Ōēż40 mg/dL for men or Ōēż50 mg/dL for women; (iii) high blood pressure, defined as systolic blood pressure Ōēź130 mmHg; and (iv) high fasting blood glucose level, defined as glucose >100 mg/dL.
REFERENCES
-
METRICS

-
- 4 Crossref
- 2,255 View
- 65 Download
- Related articles in Korean J Helicobacter Up Gastrointest Res
-
Can Helicobacter pylori Infection Reduce the Efficacy of Cancer Immunotherapy?2023 December;23(4)
Eradication of Helicobacter pylori Infection Using 7-day PCR-based Tailored Therapy2023 June;23(2)
Role of Helicobacter pylori Eradication Therapy in Patients with Functional Dyspepsia2023 June;23(2)
Why Is Regional Helicobacter pylori Eradication Treatment Report Important?2022 June;22(2)







