Impact of Helicobacter pylori Eradication on the Risk of Incident Nonalcoholic Fatty Liver Disease: A Cohort Study
Article information
Abstract
Background/Aims
Previous studies have reported an association between Helicobacter pylori (H. pylori) infection and nonalcoholic fatty liver disease (NAFLD). Our study examined whether eradication for H. pylori infection reduces the risk of incident NAFLD.
Materials and Methods
This retrospective cohort study examined 3,780 adults who had no NAFLD at baseline but were infected with H. pylori. The study population was followed from January 1995 until January 2020. H. pylori infection was determined by an H. pylori-specific IgG antibody test. Fatty liver was diagnosed by ultrasound.
Results
During a median follow-up of 7.9 years, 1,294 participants developed NAFLD. In a multivariable model adjusted for age, sex, BMI, smoking status, alcohol intake, and metabolic variables, the uneradicated (for H. pylori) group exhibited a higher risk of incident NAFLD than the eradicated group (hazard ratio [HR], 1.36; 95% CI, 1.18~1.56). The multivariable analysis also demonstrated that higher BMI, current smoking and several metabolic abnormalities were significant risk factors for NAFLD. Subgroup analyses revealed that persistent H. pylori infection correlated with an increased risk of NAFLD. H. pylori eradication was associated with a decreased risk of NAFLD development.
Conclusions
H. pylori infection may have a pathophysiological role in NAFLD development. Hence, successful eradication of H. pylori decreases the risk of incident NAFLD.
INTRODUCTION
Nonalcoholic fatty liver disease (NAFLD) is a common liver disease that is histologically similar to alcohol-related liver disease but is not associated with a history of alcohol abuse [1]. Whereas simple steatosis is a benign, non-progressive disease, nonalcoholic steatohepatitis (NASH) can progress to cirrhosis and hepatocellular carcinoma [1]. NAFLD is an important health issue worldwide, with an estimated prevalence of 20% to 30% [2]. Mounting evidence suggests that NAFLD not only affects the liver, but also contributes to systemic diseases, such as cardiovascular disease, endocrine, renal disease, and extrahepatic malignancies [2].
In addition to genetic and epigenetic factors, smoking, diets rich in carbohydrate and fat, intestinal dysbiosis, and metabolic abnormalities, such as dyslipidemia, hypertension, and high fasting blood glucose, are also widely accepted risk factors for NAFLD [3-6]. In addition, recent studies have suggested that Helicobacter pylori (H. pylori) may increase the risk of NAFLD. A recent meta-analysis by Mantovani et al. [7] showed that H. pylori infection increases the risk of NAFLD in middle-aged individuals. H. pylori also increases the risk of insulin resistance, which is an important pathogenetic factor of NAFLD; this may explain the correlation between H. pylori infection and NAFLD. Moreover, H. pylori toxins and increased intestinal permeability due to H. pylori aggravate hepatic inflammation and fibrosis [8].
It is important to note that previous studies were mainly conducted on the effects of H. pylori eradication on NAFLD, and no study has investigated whether H. pylori eradication reduces the incidence of NALFD [9,10]. We believe that this is the first cohort study to examine the effects of H. pylori eradication on the incidence of NAFLD. In this study, we investigated the longitudinal effect of H. pylori infection and determined whether eradication of H. pylori reduces the risk of NAFLD.
MATERIALS AND METHODS
1. Study population
This retrospective cohort study included asymptomatic adults, aged 20 years or older, who received a regular health check-up at the Samsung Medical Center in Seoul, South Korea between January 1995 and January 2020 (Fig. 1). Since the objective of this study was to assess the longitudinal effect of H. pylori eradication on the incidence of NAFLD, we included participants who underwent at least two health check-ups, wherein an abdominal ultrasound was taken at least 1 year apart and H. pylori status at baseline was available (n=14,231). We excluded 10,451 participants who were negative for H. pylori (n=5,966), or had fatty liver on baseline ultrasound (n=3,289), history of malignancy (n=433), history of chronic liver disease or cirrhosis (n=489), alcohol intake ≥30 g/day (n=343), or positive serologic markers for hepatitis B or C virus (n=141). Finally, 3,780 participants who were positive for H. pylori but had no features of NAFLD were included in this cohort study. This study was approved by the Institutional Review Board of the Samsung Medical Center. The board waived the requirement for informed patient consent because only de-identified data collected during the health screening visits were used.
2. Data collection
Data collection and variables have been described in detail in a previous study [11]. The comprehensive health check-up included demographic characteristics, anthropometric measurements, detailed physical examination, serum biochemical measurements, and a self-administered health questionnaire on smoking, alcohol consumption, and personal medical history. Smoking status was categorized as never, former, or current smoker. Alcohol consumption was divided into mild (≤10 g/day) and modest (>10 g/day). BMI was calculated as weight in kilograms/height in square meters (kg/m2). Blood pressure was measured in the seated position after 5 minutes of rest using an automated blood pressure monitor. Blood samples were collected in the morning after a ≥12 hours fast and analyzed by the hospital clinical laboratory. Serum levels of glucose, LDL-cholesterol (LDL-C), HDL-cholesterol (HDL-C), triglycerides, ALT, GGT, and high-sensitivity-CRP (hsCRP) were measured as part of the health check-up examination.
3. Variables
The primary outcome was incident NAFLD at follow-up, as defined by abdominal ultrasound [11]. Since we excluded participants with excessive alcohol use and/or other chronic liver disease, cases of incident fatty liver were considered NAFLD. Serum immunoglobulin G antibody to H. pylori was measured using an enzyme-linked immunosorbent assay. For potential confounders, data on age, sex, BMI, smoking status, alcohol use, metabolic variables, and liver enzymes were collected. H. pylori eradication was provided to patients, diagnosed by histologic result from stomach tissue, according to the clinical indications (history of peptic ulcer) or the patient’s presentation (asymptomatic or non-ulcer dyspepsia). The regimens for H. pylori eradication were either the standard triple therapy (proton pump inhibitor, amoxicillin, and clarithromycin) or bismuth quadruple therapy (bismuth, proton pump inhibitor, metronidazole, and tetracycline).
4. Statistical analysis
Cox regression models were used to estimate adjusted hazard ratios (HRs) with 95% CIs for NAFLD development according to the eradication for H. pylori. For H. pylori uneradicated group, the diagnosis time was considered as the start time of follow-up. For eradicated group, the time of eradication was defined as the start time of follow-up. We fitted three multivariable models with progressive degrees of adjustment for potential confounders. Model 1 was initially adjusted for age, sex, and BMI and then further adjusted for smoking status and alcohol intake in model 2. Model 3 was adjusted for metabolic variables and liver enzymes that could be important risk factors for NAFLD. The metabolic variables included systolic blood pressure, fasting blood glucose, triglycerides, LDL-C, HDL-C, and hsCRP. The liver enzymes included ALT and GGT. In addition, we calculated a KaplanMeier curve to evaluate the cumulative incidence of NAFLD between the two groups, and used the log-rank test to assess for significant difference.
Subgroup analyses were conducted to evaluate the consistency of the effect of H. pylori eradication on NAFLD. The pre-specified subgroups were defined by age (<50 vs. ≥50 years), sex (male vs. female), obesity by BMI (<25 vs. ≥25), current smoking (no vs. yes), modest alcohol intake (no vs. yes), and metabolic abnormalities (no vs. yes). Interactions between subgroups were tested using likelihood ratio tests comparing models with and without multiplicative interaction terms. All variables with a P-value <0.05 were considered statistically significant. All statistical analyses were performed using R version 3.4.3 (The R Foundation for Statistical Computing, Vienna, Austria).
RESULTS
1. Characteristics of the study population
The clinical and demographic characteristics between the participants with H. pylori infection without eradication (uneradicated group) and those who received eradication for H. pylori infection (eradicated group) are shown in Table 1. The eradicated group (49.5±7.7 years) was younger than the uneradicated group (50.2±8.4 years) (P=0.041). The proportion of smokers (23.9%) in the eradicated group was higher than that in the uneradicated group (16.9%) (P<0.001). In addition, LDL-C levels in the eradicated group were lower than in the uneradicated group (P<0.001). Other metabolic variables (blood pressure, blood glucose, HDL-C, triglycerides, and CRP) and liver enzymes (ALT and GGT) were not significantly different between the two groups.
2. The risk of NAFLD according to H. pylori eradication
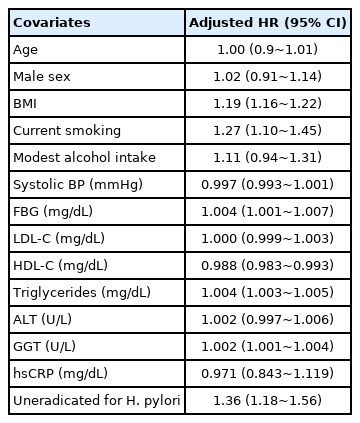
During a median follow-up of 7.9 years, NAFLD developed in 1,294 of the 3,780 participants. The uneradicated group had a higher incidence of NAFLD than the eradicated group (P<0.001) (Fig. 2). In the model 1 multivariable analysis adjusted for age, sex, and BMI, the uneradicated group (HR, 1.31; 95% CI, 1.14~1.51) exhibited a higher risk of NAFLD compared to the eradicated group. The association persisted after further adjustment for smoking status, alcohol intake, metabolic variables (systolic blood pressure, fasting blood glucose, triglycerides, LDL-C, HDL-C, and CRP), and liver enzymes (ALT and GGT) (HR, 1.36; 95% CI, 1.18~1.56) (Table 2). The multivariable analysis (Table 3) also showed that higher BMI (HR, 1.19; 95% CI, 1.16~1.22), current smoking (HR, 1.27; 95% CI, 1.10~1.45), several metabolic abnormalities (higher blood glucose level, lower HDL-C level, and higher triglycerides level), and higher GGT level were significant risk factors for incident NAFLD. Subgroup analyses also revealed that persistent H. pylori infection correlated with an increased risk of NAFLD.
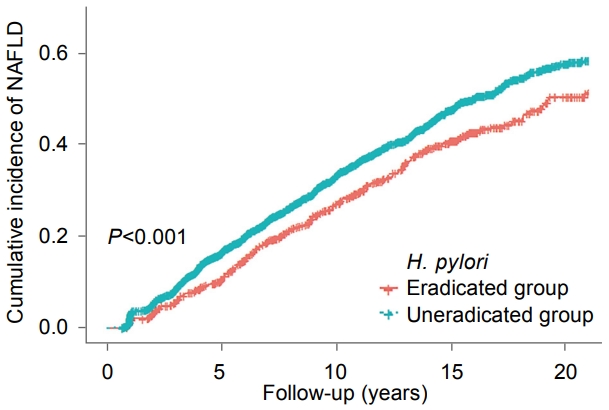
Cumulative incidence of nonalcoholic fatty liver disease (NAFLD) according to Helicobacter pylori (H. pylori) eradication.

Development of Nonalcoholic Fatty Liver Disease (NAFLD) by Helicobacter pylori (H. pylori) Eradication
3. Subgroup analyses of NAFLD risk by H. pylori eradication
Subgroup analyses were performed to evaluate the consistency of the effect of H. pylori eradication on NAFLD (Table 4). This subgroup analysis showed no heterogeneity in the risk of incident NAFLD, or significant correlations with age (<50 vs. ≥50 years), sex (male vs. female), obesity by BMI (<25 vs. ≥25), current smoking (no vs. yes), modest alcohol intake (no vs. yes), or metabolic abnormalities (no vs. yes).
DISCUSSION
In this cohort study, we assessed the effect of eradication of H. pylori infection on the incidence of NAFLD. We found that the eradication of H. pylori reduced the risk of NAFLD development. This association persisted even after adjusting for important risk factors for NAFLD, such as obesity, alcohol intake, several metabolic variables, and liver enzymes. These findings support the evidence that H. pylori contributes to the pathophysiology of NAFLD.
Many previous studies have reported an association between H. pylori infection and NAFLD. A recent meta-analysis, including 15 studies of 97,228 patients, revealed that H. pylori infection increased the risk of NAFLD [12]. Another meta-analysis of 13 observational studies reported that H. pylori infection was associated with a mild increase in the prevalence and incidence of NAFLD [7]. Two longitudinal studies reported a positive association between H. pylori infection and NAFLD. A retrospective cohort study of 17,028 participants without NAFLD at baseline suggested that H. pylori infection was associated with an increased risk of NAFLD development during a median period of 5.1 years [11]. A multi-center longitudinal cohort study of 369 participants without NAFLD at baseline demonstrated that H. pylori infection was related with an increased risk of incident NAFLD over a 2-year follow-up [13]. Another multi-center cross-sectional study of 646 patients showed that H. pylori infection was an independent risk factor for NAFLD and associated with an increased degree of steatosis [14]. Another recent study of 64 obese patients demonstrated that active H. pylori infection was independently associated with NASH, severe NASH, and the presence of fibrosis, after adjustment for important risk factors of NAFLD [15].
Given the evidence that support H. pylori as a risk factor for NAFLD, an increasing number of studies have examined the effects of H. pylori infection eradication on NAFLD. However, the results are controversial. A recent study with 64 participants showed no difference between H. pylori eradication and standard management therapy on the improvement of hepatic steatosis and liver enzyme values. However, the authors also observed that H. pylori eradication improved insulin resistance more efficiently in comparison to standard management therapy [16]. Another recent study reported that the eradication of H. pylori infection decreased lipid profile, insulin resistance, NAFLD‐liver fat score, and hepatic steatosis indices [13]. In comparison, a study with 13 patients found no long-term effects of H. pylori eradication on hepatic steatosis as assessed by magnetic resonance imaging [9]. A randomized clinical trial with 100 patients also concluded that H. pylori eradication did not result in the improvement of other indicators of NAFLD, including insulin resistance [10]. In comparison, our present study suggested that the eradication of H. pylori infection decreased the risk of incident NAFLD.
Although strong pathophysiological evidence linking H. pylori infection and NAFLD has not been fully elucidated, several potential links have been suggested to explain this association. Metabolic syndrome and insulin resistance may play an essential role in the association between H. pylori infection and NAFLD. NAFLD has been considered an early hepatic manifestation of metabolic syndrome. Many studies have also reported that H. pylori infection is associated with an increased risk of insulin resistance and metabolic syndrome [17-23]. A meta-analysis of six studies has shown that H. pylori infection is significantly associated with metabolic syndrome, as well as insulin resistance [24]. Another possible mechanism between H. pylori infection and NAFLD relates to intestinal permeability and the gut microbiota. Many studies have provided considerable evidence linking gut dysbiosis to the pathogenesis of NAFLD [6,25,26]. H. pylori infection reduces gastric acidity through gastric atrophy, resulting in subsequent small intestinal bacterial overgrowth. H. pylori invasion into the intestinal mucosa and small intestinal bacterial overgrowth may increase intestinal permeability and allow bacterial or endotoxin translocation through the portal vein and into the liver [27]. As a result, endotoxin-mediated cytokines promote hepatic inflammation response and subsequent fibrosis [28,29].
There were several limitations in this study. First, although the serologic test for H. pylori is an inexpensive mass-screening diagnostic tool, the serologic test cannot differentiate between past and current infection of H. pylori. Second, although the analysis was conducted by adjusting for several important confounding factors for NAFLD, the possibility of residual confounders, due to unmeasured covariates such as exercise and dietary factors, cannot be excluded. Finally, this study population consisted of relatively healthy men and women attending a health screening program in a single institution. A validation study with other populations and/or multiple centers is needed to make our findings more generalizable.
In conclusion, this study demonstrated that the eradication of H. pylori decreased the risk of NAFLD development. This finding supports the hypothesis that H. pylori infection plays a role in the pathophysiology of NAFLD development, and conversely, the eradication of H. pylori may reduce the risk of NAFLD. As such, the benefit of H. pylori eradication has been observed not only in the prevention of peptic ulcer disease and non-cardiac gastric adenocarcinoma, but also in the prevention of NAFLD.
Notes
No potential conflict of interest relevant to this article was reported.