 |
 |
- Search
| Korean J Helicobacter Up Gastrointest Res > Volume 23(1); 2023 > Article |
|
See editorial "Is Probiotic Supplementation Useful for Helicobacter pylori Eradication?" in Volume 23 on page 1.
Abstract
Background/Aims
The effects of probiotic supplementation on Helicobacter pylori (H. pylori) eradication therapy are not completely understood. In this study, we investigated the effects of continuous probiotic administration on eradication rates, recrudescence, and symptom response following completion of a course of H. pylori therapy.
Methods
This prospective, randomized, double-blind placebo-controlled trial was performed between June 2018 and 2020. Twohundred seventy patients who received a standard triple regimen for H. pylori eradication, were included in the study. Participants were randomized to receive a probiotic as adjunctive therapy (Enterococcus faecium 4.5├Ś108 and Bacillus subtilis 5.0├Ś107; Medilac-S┬«, Hanmi Pharmaceuticals, Seoul, Korea) or a placebo (one tablet thrice daily) for 28 days, following H. pylori eradication. Participants who showed successful eradication underwent a repeat 13C-urea breath test after 6 months.
Results
Eradication rates in the probiotic and placebo groups were 77.1% and 72.4%, respectively (P=0.48) using per-protocol analysis. Using intention-to-treat analysis, eradication rates were 67.4% and 65.9%, respectively (P=0.43). Of 149 patients who were followed-up after 6 months, four patients had recrudescence (2.7%). Recrudescence rates did not differ between the probiotic and placebo groups. Of the 76 patients who had non-ulcer dyspepsia, 60 (78.9%) showed symptom resolution after 6 months. This beneficial effect was most pronounced in patients with postprandial distress syndrome (P=0.02).
For more than 30 years, Helicobacter pylori (H. pylori) has been studied for its role in the development of gastrointestinal diseases. Eradication of H. pylori is important for the treatment and prevention of its recurrence [1-3]. The most widely used empirical H. pylori eradication regimen is clarithromycin-based standard triple therapy (STT), which adds a proton-pump inhibitor to two antibiotics (amoxicillin and clarithromycin). The eradication rate of clarithromycin-based STT has been unsatisfactory, and several efforts have been made to improve the outcomes [4,5]. Probiotic supplementation is one of them [6]. The most commonly used probiotics are microorganisms belonging to genera Bifidobacterium, Lactobacillus, Saccharomyces, and Bacillus. Certain probiotics may inhibit H. pylori. Probiotic supplementation decreases nausea, bloating and diarrhea associated with H. pylori medications and seems to improve eradication rates [7]. A meta-analysis of 10 clinical trials of the probiotic efficacy of H. pylori eradication shows that a combination of Lactobacillus- and Bifidobacterium-containing probiotics increases the overall eradication rates and reduces overall adverse events [8]. However, another meta-analysis of 33 randomized controlled trials confirms that these beneficial effects are marginal and only happen with several limited strains [9]. The authors reported that only the overall incidence of side effects was decreased in the probiotic supplementation group. Taken together, the role of probiotics in H. pylori eradication therapy is still controversial, and its effect might be overestimated.
Probiotics are usually bacteria and live microorganisms. A recent study assessed the effects of probiotic supplementation on changes in gut microbiota and antibiotic-related side effects (particularly changes in bowel habits) over time [10]. Co-administration of probiotics with an H. pylori eradication regimen appears to induce fewer changes in the gut microbiota, but post-antibiotic supplementation shows fewer adverse events (especially diarrhea). Nonetheless, the findings were not statistically significant [10]. Co-administration of antibiotics and probiotics may raise concerns that antibiotics might kill the probiotics and reverse their benefits. Some physicians suggest that probiotic therapy should be postponed until a few days after completing antibiotics, or they may prescribe taking them a few hours after taking the antibiotics. So far, studies on probiotic supplementation for H. pylori eradication therapy have been designed as a combination of probiotics and antibiotics and have only dealt with their effect on H. pylori eradication rates.
In this study, the starting point of probiotic supplementation was specially treated, and we supplied them after antibiotic therapy for H. pylori eradication. We aimed to analyze the influence of probiotics themselves on H. pylori eradication rates and recrudescence. We also investigated symptom responses after H. pylori eradication in subjects with non-ulcer dyspepsia.
This randomized double-blind prospective study was conducted at the Catholic University School of Medicine, St. VincentŌĆÖs Hospital from June 2018 to May 2020. We enrolled patients who underwent first-line eradication therapy for H. pylori. Initially, H. pylori infection status was confirmed by a rapid urease test histology (CLO┬« test; Kimberly-Clark, Draper, UT, USA), using endoscopic biopsy specimens taken from the antrum and corpus. Furthermore, we checked serum IgG for H. pylori or a 13C-urea breath test (UBT).
We measured the presence of serum IgG against H. pylori (Enzygnost┬«; Dade Behring, Marburg, Germany), and IgG titers over 15 U/mL were classified as H. pylori-seropositive in accord with the manufacturerŌĆÖs instructions. We performed a 13C-UBT with a Pranactin┬«-Citric drug product, a component of the BreathTekTM UBT Kit (Korea Otsuka Pharmaceutical Co. Ltd., Seoul, Korea). The patients ingested 3 g of reconstituted Pranactin┬«-Citric containing 75 mg of 13C-urea. After mouthwash, we collected breath samples before and 20 minutes following the administration of 13C-urea. The 13C/12C ratio in the breath sample was measured with an infrared spectrophotometer (UBiT-IR300; Korea Otsuka Pharmaceutical Co. Ltd.). Changes in the 13C values over baseline were expressed as Ōłå13C, and a positive result was defined as an increase of more than 2.5%. We also performed a PCR test. We extracted DNAs from the gastric mucosal tissues using a QIAamp DNA Mini Kit (Qiagen, Valencia, CA, USA) according to the manufacturerŌĆÖs protocol and then used the DNAs for PCR. The DNAs were stored at -200 C until use. PCR (Seeplex┬« ClaR-H. pylori ACE Detection; Seegene Institute of Life Science, Seoul, Korea) was performed to identify point mutation-containing gene fragments according to the manufacturerŌĆÖs recommendations. H. pylori infection was diagnosed if two or more tests showed positive results.
Eligible subjects for this study were H. pylori-infected patients above 19 years of age. We excluded those patients who had active peptic ulcer disease within three months, those with previous antibiotics use, those who were taking medications that might affect intestinal motility including narcotics, laxatives, and prokinetics, or those who were taking probiotics or prebiotics. We also excluded patients after gastric surgery, those with chronic illness including chronic kidney disease showing serum creatinine >1.5 mg/dL and chronic heart failure showing ejection fraction <20%, or those who were pregnant.
This study protocol was approved by the Institutional Research Ethics Board of The Catholic University of Korea (VC17MESI0238) and adhered to the Declaration of Helsinki. Written informed consent was obtained from all patients.
We defined compliance as being good (taking more than 80% of total medication) or poor by counting remaining drugs after the medication. Adverse events included abdominal pain, diarrhea, constipation, dizziness, taste perversion, headache, anorexia, nausea, vomiting, or skin rash. Adverse effect was confirmed if these symptoms disrupted the daily life.
This was a randomized, double-blind parallel-group, and placebo-controlled study. We used a computer to generate random sequences for a randomization list. Patients in each group received the same H. pylori eradication regimen. In the study, clarithromycin-based triple therapy (ilaprazole 20 mg [Noltec┬«; IL-YANG Pharmaceutical Co., Seoul, Korea], clarithromycin 500 mg [Klaricid; Abbott Laboratories, North Chicago, IL, USA], and amoxicillin 1 g [Chong Kun Dang Pharmaceutical Co., Seoul, Korea]) were administered twice daily for 14 days. All medicines were taken after breakfast and dinner. As soon as the prescribed antibacterial regimen had been completed, we randomized the subjects in a 1:1 ratio and gave them either the study products (Enterococcus faecium 4.5├Ś108 and Bacillus subtilis 5.0├Ś107; Medilac-S┬«, Hanmi Pharm., Seoul, Korea) or a placebo one tablet three times daily for 4 weeks. At least 4 weeks following the completion of treatment, a 13C-UBT confirmed H. pylori eradication.
The eradication rate was evaluated in a per-protocol (PP) analysis based on the number of patients who completed the study. Patients lost to follow-up were handled as eradication failures in the intention-to-treat (ITT) analysis.
In previous relevant studies, recrudescence is generally defined as recurrence of H. pylori infection within 1 year following eradication [11]. Six months following treatment completion, the patients confirmed to have achieved successful eradication took a second 13C-UBT to determine recrudescence. Medications related to the false negative result of 13C-UBT such as proton pump inhibitors or histamine H2 receptor antagonists were discontinued before the test.
The patients with positive results of the second UBT after 6 months were determined as recrudescence. The recrudescence rate was evaluated based on the number of patients after successful H. pylori eradication.
Following H. pylori eradication, we evaluated the symptom responses of patients. The surveys were conducted by questionnaire at the time of eradication start and 6 months following H. pylori eradication. We classified the initial symptoms into four cardinal symptom categories of functional dyspepsia according to Rome IV criteria: postprandial fullness, early satiety, epigastric pain, and epigastric burning [12]. Symptoms must have occurred at least three days a week for the preceding six months in order to be diagnosed as functional dyspepsia. The four symptoms can be classified into two subtypes: postprandial distress syndrome (PDS) or epigastric pain syndrome (EPS). According to the predominant symptom, the patientŌĆÖs symptom subtypes are initially classified. Six months following successful H. pylori eradication, we evaluated whether symptoms remained or were improved. We also analyzed the link between changes in the symptoms and recrudescence.
Student T-test was used to compare continuous variables between the two groups. Differences between dichotomous variables were evaluated with the chi-squared test. Calculation was performed with SPSS package software (SPSS version 25.0; IBM, Chicago, IL, USA). P-values less than 0.05 were considered significant.
A total of 270 patients were enrolled and received 14 days of STT as a first-line H. pylori eradication therapy. After randomization, we administered 4 weeks of probiotic supplementation to 135 patients and a placebo to other 135 patients following eradication therapy (Fig. 1). The baseline clinical characteristics of these two groups are shown in Table 1.
In the ITT analysis, there was no significant difference in the eradication rate between the two groups (probiotics vs. placebo, 67.4% vs. 65.9%; P=0.43). During follow-up periods, 17 patients in the probiotic supplementation group and 12 patients in the placebo group did not follow up. All remaining patients showed good compliance. In the PP analysis, patients in the probiotic group had a similar eradication rate compared to the placebo group (probiotics vs. placebo, 77.1% vs. 72.4%; P=0.48) (Fig. 2). All adverse events did not interfere the completion of medication. Adverse effects were spontaneously resolved or were appropriately treated by physicians.
Six months after the first UBT tests, 149 patients (75 patients in the probiotic group and 74 patients in the placebo group) who were confirmed as having successful eradication therapy, took a second UBT to evaluate whether H. pylori had recurred. Among them, four patients had recrudescence. Three patients in the probiotic group and one patient in the placebo group showed recrudescence. The recrudescence rate did not differ significantly between the two groups (4.0% vs. 1.4% P=0.62). The total recrudescence rate confirmed by UBT was 2.7%.
Initially, 36 patients in the probiotic group had dyspeptic symptoms (16 post prandial distress [PPD], 20 EPS) and 40 patients in the placebo group had dyspeptic symptoms (17 PPD, 23 EPS). Six months after the H. pylori eradication, a total of 60 patients (78.9%) had resolution of their symptoms. Among them, 29 patients belonged to the probiotic group (15 PPD, 14 EPS) and 31 patients belonged to the placebo group (15 PPD, 16 EPS). There was no significant difference in the resolution of dyspeptic symptoms between the two groups (probiotics vs. placebo, 80.5% vs. 77.5%; P=0.74) (Fig. 3). As for the PDS and EPS symptom subtypes, the beneficial effect was evident in PDS (PDS vs. EPS, 90.9% vs. 69.8%; P=0.02) (Fig. 4).
In several previous studies and meta-analyses, probiotic supplementation during standard H. pylori therapy seems to improve the eradication rate, especially in Asians [13,14]. It is also known to decrease the incidence of adverse effects. The possible mechanisms by which probiotics inhibit H. pylori infection would be through the production of bacteriocins as alternative antimicrobial agents [15-17]. By production of anti-inflammatory and anti-oxidative substances, reducing pathogenic adherences, and direct competing in the gut microbiota, multiple studies showed that probiotics inhibit H. pylori directly or indirectly [15,17]. However, probiotic supplementation did not improve the eradication rate in our study. Moreover, H. pylori-positive dyspeptic patients had marginal symptom reduction with probiotics. These results differ from previous reports.
Today, people consume probiotic supplements extensively because of increasing concerns over digestive health. Recently, in the era of the COVID-19 pandemic, easy availability of information on the internet contributes to increased attention to probiotic products, and the health benefits of probiotics have been widely discussed in the media. However, the scientific evidence of probiotic supplementation seems to be limited [18]. We need to pursue the truth and be wary of being swept away. We hypothesized that the expected beneficial effects of probiotics might be strain-specific or exaggerated. Our result shows a similar conclusion for the eradication and recrudescence. For more confirmative evaluation, big data analysis is necessary.
The eradication rate of this study was 77.1% in the probiotics group and 72.4% in the control group in the PP analysis. Although the PCR test for clarithromycin resistance was performed before eradication, the rate was below 80% and similar to previous studies in South Korea. Our regimen was clarithromycin-based triple therapy for 14 days, and the eradication rate of STT tends to decrease recently due to the increasing antibiotic resistance, especially clarithromycin. STT regimen has been recommended in the guideline for first-line treatment until recently. Probiotics supplementation is one of the attempts to recover the eradication rate, but the present data is insufficient to prove the beneficial effect of H. pylori eradication.
Recurrence of H. pylori infection can occur either as recrudescence or as reinfection [11,19]. The time window for recrudescence is generally shorter and is defined as reappearance of H. pylori infection within 1 year following eradication. In most cases, this comes from the initial false negative results of post-eradication testing. It is difficult to generalize recrudescence tendencies to whole populations from previous data because these studies are retrospective reviews [11,19]. In addition, these studies were not conducted uniformly, a decisive reason why we could not believe the purported results. In past studies, recrudescence rates have been up to 35% with dual antimicrobial therapy and approximately 15% with triple therapy [20]. Recrudescence seems to occur when eradication regimens exhibit bacteriostatic rather than bactericidal activities, suppressing rather than eradicating the organism. Previously, several investigators insisted that H. pylori modified its morphology from a spiral to coccoid form as an adaptation to harsh circumstances [21]. H. pylori can thus remain latent for a long time and possibly contribute to recrudescence. The occurrence of recrudescence decreases with time and declines sharply after the first year. Probiotics can inhibit the recrudescence of H. pylori by reducing urease activity, preventing adhesion, and inhibiting flagellar motility to induce inactive form [22,23,24].
The 13C-UBT is accurate and noninvasive. Although manufacturers provide uniform protocols, the testŌĆÖs accuracy and reproducibility have been continuously investigated. However, its reliability for assessment after eradication treatment has not been evaluated sufficiently. In addition, the cut-off value for 13C-UBT can have poor specificity for confirming H. pylori status if the patients have had multiple prior eradication therapies or moderate-to-severe gastric intestinal metaplasia [25]. In this study, we selected 2.5% as the strictest level and tried to minimize the false negative results. Our results showed that the recrudescence rate following successful eradication was 2.7%. The limitation of this analysis is that the number of revisits was reduced because of the COVID-19 pandemic and the sample size was smaller than originally planned.
A Cochrane meta-analysis of 17 randomized trials reported that H. pylori eradication exerted a small but statistically significant long-term benefit on the relief of non-ulcer dyspeptic symptoms compared to placebo [26]. The efficacy of eradication therapy was seen in all symptom subtypes of functional dyspepsia, but not uniformly among the subtypes. A trial assessing the effect of eradication therapy according to individual symptoms had a significant effect on epigastric pain and burning but not on early satiety or postprandial fullness [27]. This suggests that patients with EPS may benefit more from H. pylori eradication therapy than others. Epigastric pain partly links to hypersecretion of gastric acid and is known to be improved 6 months to 1 year following successful H. pylori eradication. In cases of postprandial discomfort, gastric accommodation could be recovered after the eradication. The beneficial symptom effect was more prominent in Westerners than Asians [26-28]. Our results also showed that the beneficial effect was more evident in PDS than EPS. In most Asian countries, H. pylori infection is more prevalent than in Western countries, and there are many patients chronically infected with H. pylori. Chronic H. pylori infection can result in severe gastric atrophy with decreased secretion of gastric acid. Such patients may not experience symptomatic improvement from H. pylori eradication.
In our study, we observed that approximately 80% of patients experienced symptom resolution after 6 months of H. pylori eradication. If this study were designed to have a control group (patients with dyspepsia who had failed to clear H. pylori), this could remove the uncertainty as to whether H. pylori eradication truly improves dyspepsia symptoms. In addition, when enrolling patients, we classified initial symptoms according to Rome IV criteria for functional dyspepsia without considering the overlap with irritable bowel syndrome. Moreover, probiotic effects were marginal among those patients with successful eradication. In this study, there was no statistically significant difference in the resolution of symptoms between the probiotic and placebo groups.
In conclusion, the overall recrudescence rate was 2.7%, and approximately 80% of patients resolved their symptoms 6 months after H. pylori eradication. Consecutive probiotic supplementation as adjunctive therapy for H. pylori eradication had marginal beneficial effects on the eradication rate, preventing recrudescence or symptom relief. We need large-scale and high-quality randomized clinical trials to establish the beneficial effects of probiotic supplementation on H. pylori-associated functional dyspepsia.
Fig.┬Ā1.
Study flowchart. H. pylori, Helicobacter pylori; F/U, follow-up; STT, clarithromycin-based standard triple therapy; ITT, intention-to-treat; PP, per-protocol.

Fig.┬Ā2.
Effects of probiotic supplementation on Helicobacter pylori eradication rates. Following completion of eradication therapy, we performed intergroup comparison between the probiotic group (participants received a probiotic supplement containing Enterococcus faecium 4.5├Ś108 and Bacillus subtilis 5.0├Ś107, Medilac-S┬« ; Hanmi Pharmaceuticals, Seoul, Korea as adjunctive therapy) and placebo group (participants received one tablet thrice daily) for 28 days. We observed no significant differences between the two groups. ITT, intention-to-treat; PPA, per-protocol analysis.
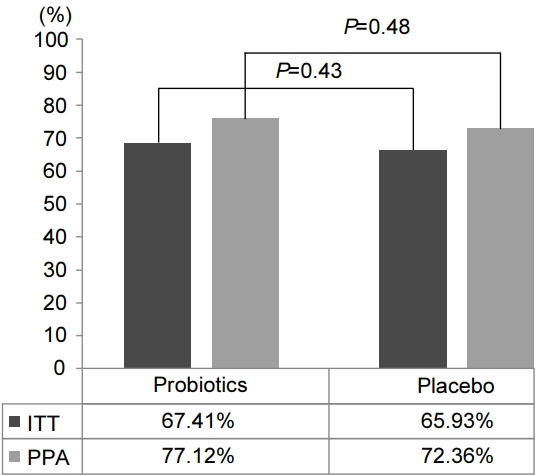
Fig.┬Ā3.
Effects of probiotic supplementation on improving dyspeptic symptoms. Comparison between the probiotic group and placebo group shows no sighificant differences. Intergroup comparison following subgroup categorization of the initial symptoms of functional dyspepsia based on the Rome IV criteria shows no significant differences. EPS, epigastric pain syndrome; PDS, postprandial distress syndrome.
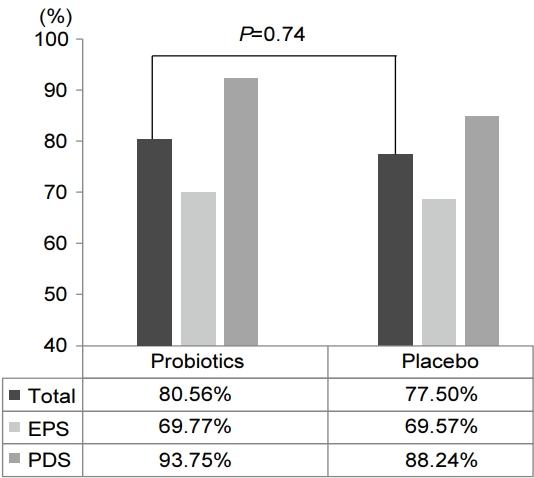
Fig.┬Ā4.
Eradication rates of Helicobacter pylori in patients with dyspeptic symptoms. Intergroup comparison following subgroup categorization of functional dyspepsia shows statistically significant improvement in patients with postprandial distress syndrome (P=0.02). EPS, epigastric pain syndrome; PPD, postprandial distress.

Table┬Ā1.
Clinical Characteristics of the Probiotic and Placebo Groups
REFERENCES
1. Chen N, Amenta PS, et al. Helicobacter pylori associated chronic gastritis, clinical syndromes, precancerous lesions, and pathogenesis of gastric cancer development. World J Gastroenterol 2014;20:5461-5473.



2. Amieva M, Peek RM Jr. Pathobiology of Helicobacter pylori-induced gastric cancer. Gastroenterology 2016;150:64-78.



3. Malfertheiner P, Megraud F, O'Morain CA, et al. Management of Helicobacter pylori infection-the Maastricht V/Florence consensus report. Gut 2017;66:6-30.


4. Boltin D, Niv Y, Sch├╝tte K, Schulz C. Review: Helicobacter pylori and non-malignant upper gastrointestinal diseases. Helicobacter 2019;24 Suppl 1:e12637.



5. Shah SC, Iyer PG, Moss SF. AGA clinical practice update on the management of refractory Helicobacter pylori infection: expert review. Gastroenterology 2021;160:1831-1841.



6. Fallone CA, Moss SF, Malfertheiner P. Reconciliation of recent Helicobacter pylori treatment guidelines in a time of increasing resistance to antibiotics. Gastroenterology 2019;157:44-53.


7. Eslami M, Yousefi B, Kokhaei P, et al. Are probiotics useful for therapy of Helicobacter pylori diseases? Comp Immunol Microbiol Infect Dis 2019;64:99-108.


8. Wang ZH, Gao QY, Fang JY. Meta-analysis of the efficacy and safety of Lactobacillus-containing and Bifidobacterium-containing probiotic compound preparation in Helicobacter pylori eradication therapy. J Clin Gastroenterol 2013;47:25-32.


9. Dang Y, Reinhardt JD, Zhou X, Zhang G. The effect of probiotics supplementation on Helicobacter pylori eradication rates and side effects during eradication therapy: a meta-analysis. PLoS One 2014;9:e111030.



10. Wang ZJ, Chen XF, Zhang ZX, et al. Effects of anti-Helicobacter pylori concomitant therapy and probiotic supplementation on the throat and gut microbiota in humans. Microb Pathog 2017;109:156-161.


11. Sun Y, Zhang J. Helicobacter pylori recrudescence and its influencing factors. J Cell Mol Med 2019;23:7919-7925.




12. Van den Houte K, Carbone F, Goelen N, et al. Effects of Rome IV definitions of functional dyspepsia subgroups in secondary care. Clin Gastroenterol Hepatol 2021;19:1620-1626.


13. Oh B, Kim BS, Kim JW, et al. The effect of probiotics on gut microbiota during the Helicobacter pylori eradication: randomized controlled trial. Helicobacter 2016;21:165-174.


14. Shi X, Zhang J, Mo L, Shi J, Qin M, Huang X. Efficacy and safety of probiotics in eradicating Helicobacter pylori: a network meta-analysis. Medicine (Baltimore) 2019;98:e15180.


15. Avonts L, De Vuyst L. Antimicrobial potential of probiotic lactic acid bacteria. Meded Rijksuniv Gent Fak Landbouwkd Toegep Biol Wet 2001;66:543-550.

16. Aiba Y, Suzuki N, Kabir AM, Takagi A, Koga Y. Lactic acid-mediated suppression of Helicobacter pylori by the oral administration of Lactobacillus salivarius as a probiotic in a gnotobiotic murine model. Am J Gastroenterol 1998;93:2097-2101.


17. Kamiya S, Yonezawa H, Osaki T. Role of probiotics in eradication therapy for Helicobacter pylori infection. Adv Exp Med Biol 2019;1149:243-255.


18. Suez J, Zmora N, Segal E, Elinav E. The pros, cons, and many unknowns of probiotics. Nat Med 2019;25:716-729.



19. van der Ende A, van der Hulst RW, Dankert J, Tytgat GN. Reinfection versus recrudescence in Helicobacter pylori infection. Aliment Pharmacol Ther 1997;11 Suppl 1:55-61.

20. Xia HX, Gilvarry J, Beattie S, et al. Recrudescence of Helicobacter pylori infection in patients with healed duodenal ulcer after treatment with different regimens. Am J Gastroenterol 1995;90:1221-1225.

21. Reshetnyak VI, Reshetnyak TM. Significance of dormant forms of Helicobacter pylori in ulcerogenesis. World J Gastroenterol 2017;23:4867-4878.



22. Boonyaritichaikij S, Kuwabara K, Nagano J, Kobayashi K, Koga Y. Long-term administration of probiotics to asymptomatic pre-school children for either the eradication or the prevention of Helicobacter pylori infection. Helicobacter 2009;14:202-207.


23. Martinez RC, Bedani R, Saad SM. Scientific evidence for health effects attributed to the consumption of probiotics and prebiotics: an update for current perspectives and future challenges. Br J Nutr 2015;114:1993-2015.


24. Chen YH, Tsai WH, Wu HY, et al. Probiotic Lactobacillus spp. act against Helicobacter pylori-induced inflammation. J Clin Med 2019;8:90.



25. Kwon YH, Kim N, Lee JY, et al. The diagnostic validity of citric acid-free, high dose (13)C-urea breath test after Helicobacter pylori eradication in Korea. Helicobacter 2015;20:159-168.


26. Moayyedi P, Soo S, Deeks J, et al. Eradication of Helicobacter pylori for non-ulcer dyspepsia. Cochrane Database Syst Rev 2006;CD002096.


-
METRICS

-
- 1 Crossref
- 1,442 View
- 56 Download
- Related articles in Korean J Helicobacter Up Gastrointest Res
-
Antibiotic Resistance and Helicobacter pylori Eradication Therapy2023 September;23(3)
Eradication of Helicobacter pylori Infection Using 7-day PCR-based Tailored Therapy2023 June;23(2)
Is Probiotic Supplementation Useful for Helicobacter pylori Eradication?2023 March;23(1)
Endoscopic Scoring System for Predicting Helicobacter pylori Infection2022 December;22(4)






