Gastric Mucosa-associated Lymphoid Tissue Lymphoma Mimicking Signet Ring Cell Carcinoma
Article information
Abstract
Gastric mucosa-associated lymphoid tissue (MALT) lymphoma is the most common type of extranodal non-Hodgkin lymphoma. Endoscopic findings are nonspecific and variable; therefore, differentiation of this malignancy from early gastric cancer is challenging during endoscopy. Although an endoscopic biopsy is the gold standard for diagnosis, a biopsy may not conclusively establish the diagnosis in all cases. Diagnostic confirmation requires interpretation of the biopsy specimen findings by an experienced histopathologist, and an additional immunoglobulin heavy chain (IgH) rearrangement test may aid with accurate diagnosis. We present a case of gastric MALT lymphoma that histopathologically mimicked signet ring cell carcinoma (SRCC) on evaluation of repeat endoscopic biopsies. Following endoscopic submucosal dissection (ESD), we confirmed the final diagnosis of gastric MALT lymphoma based on histopathological findings of prominent lymphoid infiltrates accompanied by lymphoepithelial lesions and results of the monoclonal IgH rearrangement test. Notably, a few carcinoma-like signet ring cells (SRCs) in the specimen were attributed to a reactive change. Clinicians should be mindful of possible SRCs in gastric MALT lymphoma specimens to avoid misdiagnosis of SRCC in patients with gastric MALT lymphoma. Confirmatory ESD may be useful for accurate diagnosis and appropriate management of such lesions.
INTRODUCTION
Gastric mucosa-associated lymphoid tissue (MALT) lymphoma is the most common extra-nodal non-Hodgkin lymphoma, representing approximately 5% of primary gastric neoplasia [1,2]. As most patients are asymptomatic or present nonspecific complaints, gastric MALT lymphoma is usually diagnosed following endoscopy performed for routine screening or dyspepsia evaluation. Gastric MALT lymphoma most often involves the antrum, but can occur any part of the stomach and the distribution is usually multifocal [3,4]. Since endoscopic findings are nonspecific and variable, it is often difficult to differentiate it from benign Helicobacter pylori (H. pylori)-associated lesions or early gastric cancer (EGC) during endoscopy. Clinical suspicion of gastric MALT lymphoma during an endoscopic examination is the first step for the diagnosis, and an endoscopic biopsy is the gold standard for diagnosing gastric MALT lymphoma [5]. Adequate tissue sampling with multiple and deep biopsies is essential, and the specimen should be interpreted by an experienced pathologist. Although Wotherspoon’s histologic scoring system is used to make a confident diagnosis of gastric MALT lymphoma, false-negative results are possible and there are still occasions which is difficult to determine an accurate diagnosis despite endoscopic repeated biopsies [6]. Here, we present a case of gastric MALT lymphoma histologically mimicking signet ring cell carcinoma (SRCC) on endoscopic repeated biopsies.
The Institutional Review Board of Pusan National University Hospital reviewed and approved this study (IRB No. 2303-010-124).
CASE REPORT
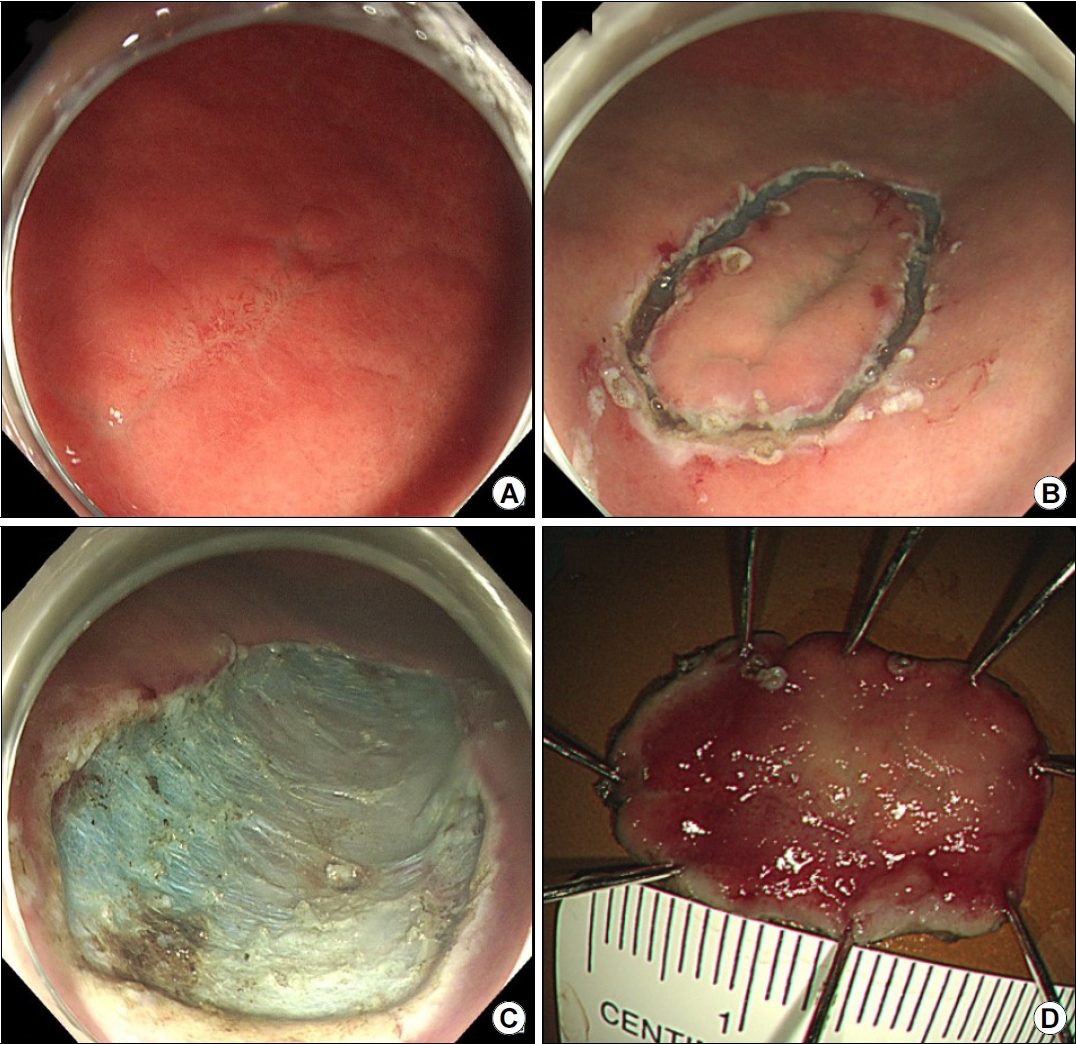
A 38-year-old man was referred to our gastroenterology clinic for further evaluation of the discolored gastric lesion detected on a screening esophagogastroduodenoscopy (EGD). On EGD at local clinic, there was a 1.5 cm-sized slightly depressed white discolored mucosa on greater curvature of angle (Fig. 1A). Five endoscopic biopsy pieces were obtained, and the biopsy sample showed some groups of atypical cells with signet ring cell (SRC) features on the background of marked lymphocytic infiltration, with the possibility of poorly differentiated adenocarcinoma (Fig. 2A, B). He had no gastrointestinal symptoms, and physical examination and laboratory tests were normal. Repeated endoscopy with EUS was done at our department at an interval of 16 days after the first examination at local clinic. There was biopsy-induced erythematous mucosal change on the original discolored lesion (Fig. 1B), and EUS revealed a markedly homogeneous hypoechoic lesion involving 2nd and 3rd layers (Fig. 1C). Endoscopic and EUS features were suspicious of gastric MALT lymphoma rather than EGC, and repeated endoscopic biopsy was done. Three pieces were taken, and the biopsied tissue showed dense lymphocyte infiltration with a few SRC-like atypical cells as well as lymphoepithelial lesions (LELs) (Fig. 2C, D). Clinical suspicion based on the endoscopic features and pathologic findings after repeated endoscopy was gastric MALT lymphoma, but we could not clearly interpret the presence of SRCs within lymphocyte infiltration. Abdominal CT revealed no abnormal findings, and in order to rule out the possibility of SRCC, endoscopic submucosal dissection (ESD) was done (Fig. 3). The final diagnosis was gastric MALT lymphoma based on the prominent lymphoid stroma with LELs and monoclonality of immunoglobulin heavy chain (IgH) gene rearrangement test, while a few epithelial cells showing SRC-like change was considered reactive change (Fig. 4A-D). Immunohistochemical (IHC) study revealed positive for CD20 and Bcl-2, but negative for CD3 and Bcl-6 (Fig. 4E-H). Rapid urease test was positive, and H. pylori eradication was successfully done after ESD. Six months later follow-up study including EGD and abdominal CT showed no evidence of disease recurrence.
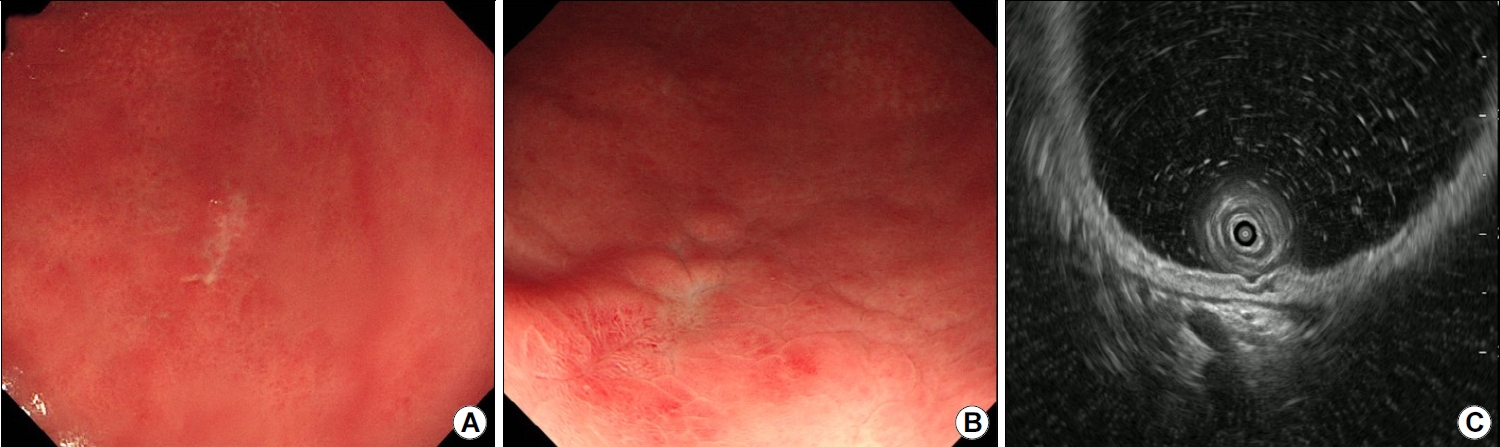
(A) Initial endoscopy findings showing a slightly depressed area of white discoloration (1.5 cm in size) along the greater curvature of the angle. (B) Repeat endoscopy findings showing erythematous change in the discolored mucosa after a previous forceps biopsy specimen was obtained from the site. (C) EUS scan showing a prominently homogeneous hypoechoic lesion involving the 2nd and 3rd layers.

(A, B) Findings in an initial biopsy specimen showing significant lymphocytic infiltration (H&E, ×40) and some groups of signet ring cells, which are associated with a high index of clinical suspicion for signet ring cell carcinoma (H&E, ×200). (C, D) Repeat biopsy specimen showing dense lymphocytic infiltration (H&E, ×40) and a few signet ring cells, as well as lymphoepithelial lesions (H&E, ×200).

(A) ESD specimen findings showing foci of heavy lymphocytic infiltration with lymphoid follicles (H&E, ×12.5). (B) Dense lymphocytic infiltrates with lymphoepithelial lesions are observed (H&E, ×100). (C) Some epithelial cells showing signet ring cell-like characteristics (H&E, ×200). (D) Immunohistochemical analysis for pancytokeratin showing highlighted lymphoepithelial lesions. The lymphoid cells are immunopositive for CD20 (E) and Bcl-2 (F) but immunonegative for CD3 (G) and Bcl-6 (H) (D-H: magnification ×100). ESD, endoscopic submucosal dissection.
DISCUSSION
Endoscopic findings of superficial-type gastric MALT lymphoma are nonspecific and variable including erythema, erosion, ulcer, discoloration, or polypoid, therefore it is often difficult to differentiate it from benign H. pylori-associated lesions or EGC during endoscopy. Especially when the lesion is solitary and IIc-like type, it is sometimes hard to differentiate gastric MALT lymphoma from EGC. Several differential points of endoscopic features between gastric MALT lymphoma and EGC have been suggested [7]. Compared to EGCs, gastric MALT lymphomas are relatively glossy with cobblestone appearance, and the border is usually indistinct with convexly or concavely tapered mucosal folds. Although EUS is generally applied for evaluating the tumor depth and predicting the treatment response of H. pylori eradication, it also can be helpful to differentiate gastric MALT lymphoma from EGC [8]. Markedly homogeneous hypoechoic lesion is the key finding of lymphoid infiltrate, which is more hypoechoic than that of EGC. In the diagnosis of gastric MALT lymphoma, clinical suspicion during an endoscopic examination is critical, and an endoscopic biopsy is the gold standard. Currently, Wotherspoon’s histologic scoring system is used to make a confident diagnosis of gastric MALT lymphoma, and grade 5 showing dense diffuse infiltrate of centrocyte-like cells in the mucosa with prominent LELs is conclusive for the diagnosis. In cases of grade 3 or 4, suspicious of gastric MALT lymphoma, monoclonality of B cells on IgH gene rearrangement test can confirm the diagnosis [6]. Since the tumor cells of gastric MALT lymphoma originates from the deep mucosa or submucosa without destroying the foveolar glands, false-negative results are not uncommon. Therefore, sufficient tissue sampling with multiple and deep biopsies is essential for improving the diagnostic yield, and the specimen should be interpreted by an experienced pathologist for an accurate diagnosis [5]. Diagnostic ESD might be considered when gastric MALT lymphoma is strongly suspected but missed for accurate and timely diagnosis and subsequent treatment [9].
In our case, initial endoscopic biopsy was highly suspicious of SRCC. However, endoscopic repeated biopsy revealed dense lymphoid infiltration with LELs which is suggestive of gastric MALT lymphoma, while the presence of SRCs confused us to conclude a final diagnosis. Additionally, singleness of the lesion was troublesome to differentiate the lesion between gastric MALT lymphoma and SRCC, though endoscopic and EUS feature were close to gastric MALT lymphoma. Although additional IHC study using biopsy specimen would provide a clue for the diagnosis of gastric MALT lymphoma, we thought that it still could not clearly define the presence of SRCs. In this situation, we determined that a histological evaluation of the entire lesion through ESD was necessary for an accurate diagnosis and further treatment, and therefore we could make sure the diagnosis of gastric MALT lymphoma and determine the SRC-like change as reactive after ESD.
Actually, histologically distinguishing gastric MALT lymphoma from adenocarcinoma is not that hard in clinical practice, though false-negative results are common as mentioned above. As in the present case, several cases have been reported showing SRCs in gastric MALT lymphoma. Zamboni et al. [10] noticed the presence of epithelial SRCs in a proportion of primary gastric B-cell lymphomas, and in some endoscopic biopsies, they found it difficult to decide whether they represented an associated carcinoma. Therefore, they reviewed 108 stomachs resected for primary lymphoma including 70 MALT lymphoma, and found SRCs, either isolated or grouped in clusters, in 26 of 70 MALT lymphomas. They suggested that SRCs in gastric MALT lymphoma represent a particular type of LEL in which the foveolar cells disaggregated by the lymphomatous infiltration acquire a globoid, signet-ring appearance. Regarding this study, it seems SRCs are not rare in gastric MALT lymphoma. However, we have to sense that the previous study results were based on the entire review of the resected stomachs and the lesions might be more advanced than those in currently diagnosed cases. Since superficial-type gastric MALT lymphoma is common nowadays and the current diagnosis is generally made by endoscopic biopsy samples, the prevalence of SRCs in gastric MALT lymphoma is supposed to be lower than that of the previous report in current practice. A cell with signet ring morphology is not always malignant [11]. There are benign SRC changes that can mimic SRCC such as vacuolization of the foveolar epithelium, hyperplastic polyp with globoid change, glassy cell change, prominent mucus neck cells, or ischemic/autolytic change. Apart from benign diseases, malignant tumors including lymphoma, poorly differentiated intestinal gastric adenocarcinoma, neuroendocrine tumor, metastatic lobular breast cancer, or ovarian cancer can have SRC change. In gastric MALT lymphoma, carcinoma-like SRCs can be seen, as multiple or single clusters, intermingled with diffuse infiltrate of marginal-zone B-cells.
In conclusion, as SRC morphology can be present in gastric MALT lymphoma, it is important to avoid a misdiagnosis of SRCC in gastric MALT lymphoma. An experienced review of histology accounting for the endoscopic and EUS features is necessary for an accurate diagnosis. In cases which still have a diagnostic uncertainty, confirmative ESD should be considered for the proper management of such lesions. Future research on a prevalence of SRCs in gastric MALT lymphoma using endoscopic biopsy samples can help understand the real world practice.
Notes
There is no potential conflict of interest related to this work.