Risk Factors for Metachronous Recurrence after Endoscopic Submucosal Dissection of a Gastric Neoplasm
Article information
Abstract
Background/Aims
Although endoscopic submucosal dissection (ESD) is an accepted treatment method for gastric neoplasm worldwide, metachronous recurrence often occurs. Here, we evaluated the risk factors for metachronous recurrence after ESD of gastric dysplasia or adenocarcinoma and also examined the effects of Helicobacter pylori (H. pylori) eradication.
Materials and Methods
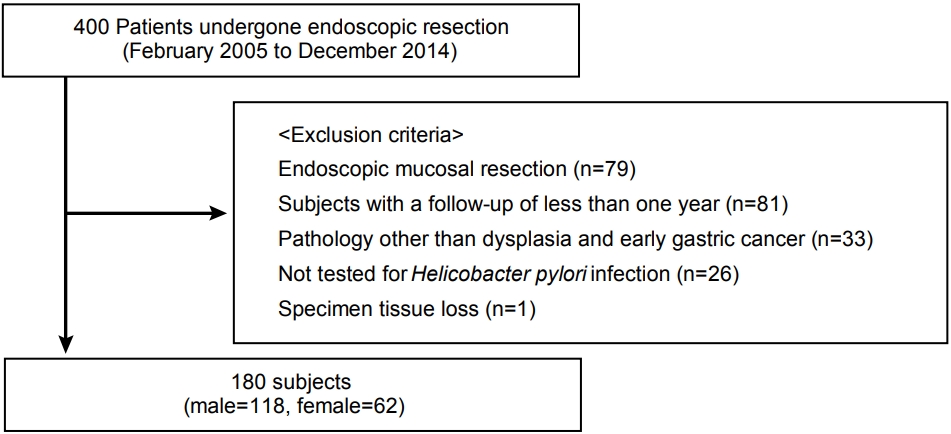
Among 400 patients who underwent endoscopic resection from February 2005 to December 2014 at Ewha Womans University Hospital, the medical records of 180 patients were retrospectively reviewed.
Results
The enrolled patients included 118 men and 62 women, and their median age was 61.7±10.3 years. During a median follow-up period of 34.5 months, metachronous recurrence occurred in 21 (11.7%) patients. Multivariate analyses revealed that H. pylori eradication did not have any preventive effects on metachronous recurrence. A family history of gastric cancer was the only risk factor for metachronous recurrence after ESD of the gastric neoplasm.
Conclusions
Metachronous recurrence was found to be related to family history of gastric cancer. However, H. pylori eradication had no preventive effects on metachronous recurrence after ESD of a gastric neoplasm. Therefore, intensive surveillance is required for patients who undergo ESD of a gastric neoplasm and have a family history of gastric cancer.
INTRODUCTION
Gastric cancer is the second most common malignant tumor and the third leading cause of cancer mortality in Korea [1]. Recently, the detection rate of early gastric cancer (EGC) has significantly increased owing to advances in endoscopic technology and regular cancer screening program [2,3]. EGC is a gastric cancer that invades no deeper than the submucosa, regardless of lymph node metastasis [4]. Currently, endoscopic submucosal dissection (ESD) has become the main treatment strategy for EGC resection and provides safer and curative outcomes [5-7].
The general indications for ESD are the presence of a mucosal tumor, measuring <20 mm in diameter and having differentiated histology but lacking both ulceration and visible lymphovascular invasion [8]. A previous study revealed that long-term clinical outcomes such as local recurrence, metachronous recurrence, overall survival rate, disease-free survival, and adverse events after ESD were comparable to those after surgical treatment of EGC, including the expanded indications [9,10]. ESD was superior than surgical resection in terms of higher quality of life, minimal invasiveness, organ preservation, and shorter hospital stay [11]. However, stomach preservation can increase the risk of local and metachronous recurrence, which means that these patients will need to undergo surgical or endoscopic resection (ER) [12]. According to the literature, the incidence of metachronous recurrence over a long-term period after ESD was reportedly 6.1%, whereas that after partial gastrectomy was approximately 0.7% [13]. It is critical to explain the potential risk factors for metachronous recurrence in patients who undergo ESD.
Although methods used to analyze risk factors for metachronous recurrence vary among studies, older age, multiple initial EGCs, family history of gastric cancer, and persistent Helicobacter pylori (H. pylori) infection are considered as common risk factors. Several studies have focused on H. pylori infection as a modifiable risk factor for metachronous recurrence. However, whether the eradication of H. pylori could prevent metachronous recurrence after ESD of gastric neoplasm still remains controversial [14]. The aim of this study was to analyze the risk factors for metachronous recurrence among patients who underwent ESD for gastric neoplasm as well as to investigate the preventive effects of H. pylori eradication on metachronous recurrence among these patients.
MATERIALS AND METHODS
1. Patients and study design
We retrospectively reviewed the medical records from February 2005 to December 2014. A total of 400 patients diagnosed with gastric neoplasm who underwent ER were enrolled at Ewha Womans University Medical Center, Seoul, Korea. The primary and metachronous lesion of gastric neoplasm included dysplasia and carcinoma. We excluded 79 patients who underwent endoscopic mucosal resection, 81 patients who were followed up for <1 year, 33 patients who had a pathology other than that of adenoma and EGC, 26 patients who were not examined for H. pylori infection, and one patient whose tissue sample was missing. Therefore, a total of 180 patients participated in this study (Fig. 1). Clinical pathological variables, such as age, sex, smoking status, tumor location (upper: cardia and fundus, middle: body and angle, and lower: antrum and prepylorus), endoscopic gross appearance (elevated, flat, and depressed), tumor size, histological examination results, invasion depth, H. pylori infection status, follow-up duration, and family history of gastric cancer among first-degree relatives were determined based on medical records. The study was allowed by the Ethics Committee of the Ewha Medical Research Institute (IRB number: 2019-02-006) and was implemented in compliance with the Declaration of Helsinki.
2. Detection of H. pylori infection and its eradication
Infection with H. pylori was regarded as positive when at least one positive result was obtained in urease breath test (Otsuka®, Tokyo, Japan), rapid urease test (CLOtest®; Delta West, Bentley, Australia), or histologic assessment (modified Giemsa staining) conducted using gastric biopsies from the antrum and greater curvature of the body. H. pylori eradication therapy was performed based on Korean guidelines for H. pylori infection [15]. The first-line treatment included 1,000 mg amoxicillin, 500 mg clarithromycin, and a standard dose of proton-pump inhibitor (PPI) taken twice a day for a week. If this treatment failed, a second-line regimen was prescribed for a week. This regimen comprised four drugs: 250 mg metronidazole (three times a day), 500 mg tetracycline (four times a day), 240 mg bismuth (four times a day), and a standard dose of PPI (twice a day). Either the urease breath test, rapid urease test, or Wright-Giemsa staining of gastric biopsy samples was performed to confirm successful H. pylori eradication.
3. Definition of metachronous recurrence
The diagnostic criteria for metachronous recurrence proposed by Warren [16] were used to categorize synchronous and metachronous tumors. Generally, metachronous gastric tumor is defined as gastric tumor located distant from the original tumor over a 1-year period after ER. Gastric tumor detected within 1 year after ER should be regarded as a previously missed synchronous gastric tumor.
4. Endoscopic submucosal dissection technique and follow-up
All ESD procedures were performed by two expert endoscopists (KNS, HKJ) by employing an insulated-tip knife (KD-611L; Olympus Medical, Tokyo, Japan) or Dual knife (KD-650; Olympus Medical). After the lesion was examined, argon plasma coagulation was performed in which a perimeter of 5 mm enclosing the lesion with several spots was marked using the tip of a probe. Normal saline mixed with indigo carmine and diluted epinephrine (1:100,000) was injected to lift the mucosal layer. A circumferential mucosal incision was performed outside the marking dots. The submocosa connective tissue was dissected using electrosurgical knife. This procedure is concluded on achievement of hemostasis. Follow-up endoscopic examination was scheduled at 3, 6, and 12 months post-ESD as well as on an annual basis subsequently to evaluate the completeness of resection and to detect metachronous recurrence. Biopsy samples were taken from the post-ESD scar or other suspicious mucosal abnormalities. H. pylori status was evaluated by performing rapid urease test and Wright-Giemsa staining of gastric biopsy samples at all follow-up examinations. Abdominal computed tomography was performed annually to assess distant metastasis.
5. Statistical analysis
Baseline data were obtained from our retrospectively collected medical records. Most variables, such as age and tumor size, were expressed as the mean±standard deviation. Chi-square test and Fisher’s exact test were applied to compare metachronous recurrence. Student’s t-test was used for performing intergroup comparisons of non-categorical clinical pathological variables. A univariate Cox proportional hazards model was used to identify possible covariates as significant risk factors for metachronous recurrence. Then, the variables with a P-value of <0.05 were subjected to multivariate Cox proportional hazards model to identify their independent contribution. Additionally, the variables considered to be possible risk factors for metachronous recurrence based on previous researches were also used in a multivariate model. All results were considered statistically significant at P-values of <0.05. Statistical analyses were performed using the Statistical Package for Social Science Version 18.0 (SPSS Inc., Chicago, IL, USA).
RESULTS
1. Baseline characteristics
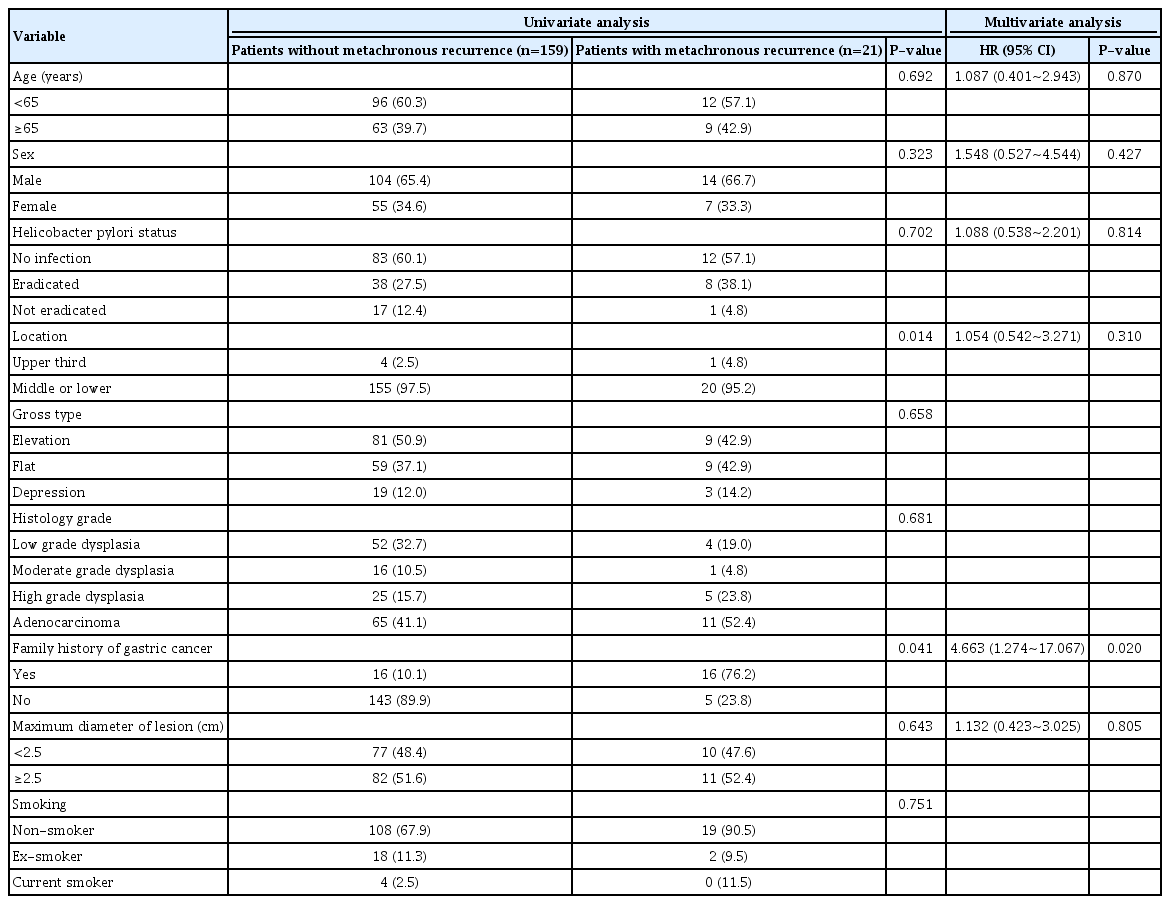
A total of 180 patients who underwent ER of gastric neoplasm were enrolled. Baseline clinicopathologic characteristics of the patients were shown in Table 1. The enrolled patients included 118 males and 62 females, and their median age was 61.7±10.3 years. During the follow-up period (median: 34.5 years, range: 11~121 years), metachronous recurrence was observed in 21 patients (11.7%). There were no statistically significant differences between patients without and with metachronous recurrence in terms of sex, smoking history, location, and gross appearance as well as the diameter, histology, or the depth of tumor. However, patients with metachronous recurrence had a significantly higher family history of gastric cancer (P=0.041).

Comparison of Clinicopathological Characteristics between Patients with and without Metachronous Recurrence
The mean age of 21 patients with metachronous recurrence was 62.3±14.6 years, and 14 (66.7%) of the patients were males. The average tumor size was 2.5 cm (range: 1.0~4.0 cm) in diameter. Of 21 lesions, one (4.8%), 11 (52.4%), and nine (42.8%) lesions developed in the upper, middle, and lower third of the stomach, respectively. Sixteen patients (76.2%) had a family history of gastric cancer. The median time to recurrence was 48.4 months (range: 6~121 months). Metachronous recurrence consisted of adenocarcinoma (14 patients, 8.8%) and dysplasia (seven patients, 4.4%), respectively. Except for the eight patients who were transferred to another hospital, all metachronous recurrence were treated by operation (four patients, 30.8%) or ESD (nine patients, 69.2%).
2. Risk factors for metachronous recurrence
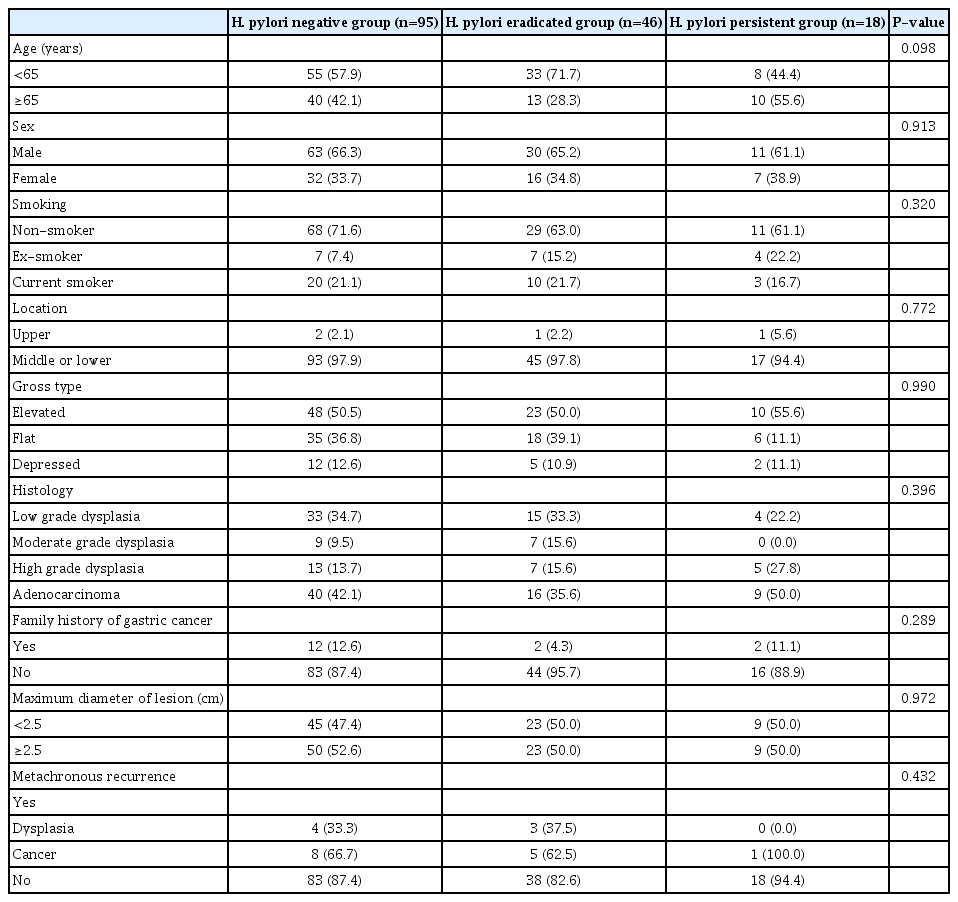
According to univariate analysis, there were statistically significant differences in tumor location (P=0.014) and family history of gastric cancer (P=0.041) between patients with metachronous recurrence and those without metachronous recurrence. Family history of gastric cancer was the only independent risk factor for metachronous recurrence (OR=4.663, 95% CI: 1.274~17.067, P=0.020) in multivariate analysis. However, H. pylori eradication was not associated with metachronous recurrence after ESD of gastric neoplasm was performed (Table 2). With regard to the patients with metachronous recurrence, there were 12 patients in the H. pylori-negative group (12/95, 12.6%), eight patients in the H. pylori-eradicated group (8/46, 17.4%), and one patient in the H. pylori-persistent group (1/19, 5.2%). The incidence of metachronous recurrence was higher in the H. pylori-eradicated group than that in the other groups without statistical significance (P=0.432). The median interval between ESD and metachronous recurrence was 48.4 (range: 12~121) months (i.e., 46.7 months in the H. pylori-negative group, 54.1 months in the H. pylori-eradicated group, and 44.4 months in the H. pylori-persistent group). There were no significant differences in the baseline clinicopathologic characteristics of patients with metachronous recurrence among the groups (Table 3).
DISCUSSION
ESD has now been widely accepted as a useful, standard treatment for certain EGCs worldwide. ESD offers minimally invasive treatment at a lower cost, but metachronous recurrence after ESD is still a matter of concern. Risk factors for metachronous recurrence after ER of gastric neoplasm were male sex, older age, multiple initial EGCs, family history of gastric cancer, and persistent H. pylori infection [15,16]. The present study shows that the family history of gastric cancer could play an important role in metachronous recurrence after ESD is performed for gastric neoplasm. However, H. pylori eradication after ESD did not significantly reduce the incidence of metachronous recurrence.
Our analysis reported a metachronous recurrence rate of 11.7% after ESD for gastric neoplasm is performed. Other studies have shown an incidence rate of metachronous recurrence ranging from 2.7% to 15.6% [17-20]. Taking the short follow-up period into account, the prevalence of metachronous recurrence in the present study is comparatively higher. With regard to metachronous recurrence, a large-population retrospective study reported that the 5-year, 7-year, and 10-year cumulative incidence rates of metachronous recurrence were 9.5%, 13.1%, and 22.7%, retrospectively [18]. A previous study reported that the risk of metachronous recurrence increased at a proportion of >5‐year follow‐up duration, regardless of the presence of H. pylori [21]. Continuous endoscopic surveillance after ESD is important to prevent metachronous recurrence.
After making adjustments during statistical analysis, a family history of gastric cancer was found to be an independent risk factor for metachronous recurrence. In this study, a history of gastric cancer in first-degree relatives increased the risk of metachronous recurrence by approximately four-fold. Although most gastric neoplasms are sporadic, approximately 10% show familial aggregation [22]. The risk of gastric neoplasm in patients with a family history is higher than the risk associated with other solid cancers [23]. First, family members share some common etiological factors such as exposure to carcinogens, dietary habits, and virulence of bacteria including H. pylori as well as common genetic backgrounds [24], and second, family history of gastric neoplasm increases the susceptibility to gastric carcinogenesis [25-28].
Family history of gastric cancer has been known as a risk factor of gastric neoplasm [29]. Our findings revealed that patients with metachronous recurrence are more likely to have a family history of gastric cancer. Similarly, a study by Kim et al. [30] reported that family history of gastric cancer (hazard ratio 2.60, P=0.014) was related to metachronous gastric neoplasm development. Although EGC has increased the number of patients undergoing ESD, there is no established guideline for effective follow-up after ESD. If the risk of metachronous recurrence of gastric cancer is high in patients who undergo ESD, it is preferable to subject them to a more intensive follow-up than that done for the general population without any risk factors for recurrence. Our results indicated that patients with a family history of gastric cancer have a high risk of metachronous recurrence. This group will benefit from a more frequent endoscopic follow-up schedule after ESD treatment.
H. pylori infection is considered to be one of the most important risk factors for gastric neoplasm. However, it remains controversial whether H. pylori eradication actually suppresses metachronous recurrence after ESD. Our study shows that H. pylori eradication after ESD of gastric neoplasm did not significantly reduce the incidence of metachronous recurrence. In a recent randomized controlled trial, H. pylori eradication did not produce significant changes in molecular alterations related to carcinogenesis, suggesting that H. pylori treatment may not prevent metachronous recurrence in the background mucosa with intestinal metaplasia [31]. The molecular changes associated with carcinogenesis have not been improved by H. pylori eradication, which means that the eradication does not prevent metachronous recurrence after ESD. As opposed to our study, several retrospective studies have considered persistent H. pylori as a risk factor for metachronous recurrence after ESD [32-34]. H. pylori eradication causes the disappearance of neutrophilic infiltration in the gastric mucosa, which in turn results in the reduction of lymphocyte and plasma cell infiltration in the gastric mucosa [35]. H. pylori eradication exerts a preventive effect on intestinal metaplasia and gastric atrophy [36]; consequently, the occurrence of metachronous recurrence in patients who receive endoscopic treatment can be reduced. A recent double-blind, randomized trial [37] was designed to evaluate whether H. pylori treatment prevents metachronous recurrence and decreases histologic changes in patients with EGC; in that study, it was revealed that as opposed to patients who received placebo, H. pylori treatment resulted in lower incidence rates of metachronous recurrence and more improvement from baseline in gastric atrophy grade. However, H. pylori treatment did not affect the subsequent incidence of adenoma or overall survival.
Several limitations of this study warrant discussion. First, it was performed at a single center and had a retrospective design. This could induce selection bias with regard to the generalization of results. The number of subjects in the H. pylori-persistent group was substantially smaller than that in the H. pylori-eradicated group. Second, the number of patients is small and the follow-up period is short for confirming the effect of H. pylori eradication on metachronous recurrence. Finally, atrophy and intestinal metaplasia in the background mucosa were not histologically evaluated. The serum pepsinogen I/II ratio or the degree of gastric mucosal atrophy during histological evaluation had to be confirmed.
In conclusion, H. pylori eradication was not found to be associated with metachronous recurrence after ESD of gastric neoplasm. However, the development of metachronous recurrence might be affected by family history of gastric cancer. Therefore, intensive surveillance is required for patients having a family history of gastric cancer and who undergo ESD of gastric neoplasm.
Notes
No potential conflict of interest relevant to this article was reported.