Tree-like Vessels on Magnifying Endoscopy
Article information
Question: A 54-year-old man underwent esophagogastroduodenoscopy (EGD) as a part of the national screening program for gastric cancer. No specific symptoms and signs were present. EGD showed multiple <1 cm-sized nodular changes on the posterior wall of the gastric upper body (Fig. 1A). Magnifying endoscopy with narrow-band imaging (ME-NBI) revealed abnormal microvessels which resembled long, bare branches of a tree in the shiny mucosa and loss of pit structures (Fig. 1B). Targeted biopsy specimens were taken from the area where abnormal microvessels were present.
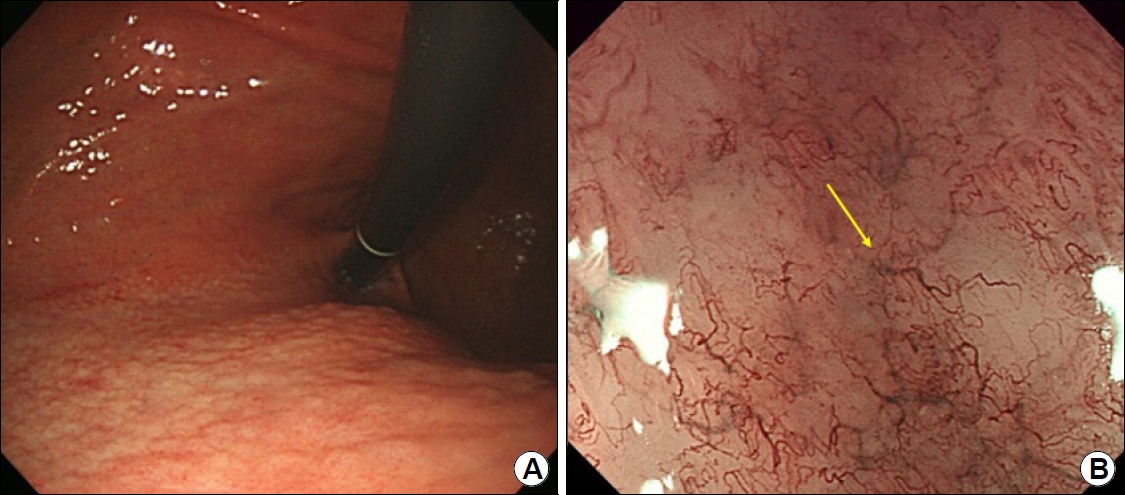
(A) Conventional endoscopy showing multiple sub-cm-sized nodular changes in the posterior wall of the gastric upper body. (B) Magnifying endoscopy with narrow-band imaging reveals abnormal microvessels (yellow arrow) resembling long, bare tree branches in the shiny mucosa (tree-like appearance) and loss of pit structures.
What is the most likely diagnosis?
Answer: Endoscopic biopsy revealed atypical lymphocyte hyperplasia with germinal centers in the lamina propria. Some clusters of atypical lymphocytes infiltrated the gastric glands, suggesting the presence of lymphoepithelial lesions (Fig. 2A). The atypical lymphocytes were positive for CD20 immunostaining (Fig. 2B). These findings were consistent with extranodal marginal zone B-cell lymphoma. The Helicobacter pylori (H. pylori) polymerase chain reaction test (Seeplex® H. pylori-ClaR ACE Detection; Seegene Inc., Seoul, Korea) result was positive, and there was no point mutation of A2142G/A2143G. The patient was treated with triple therapy composed of lansoprazole, amoxicillin, and clarithromycin for 7 days. The urea breath test (UBiT-IR300®; Otsuka Electronics Co. Ltd., Tokyo, Japan) result was negative 4 weeks after treatment completion. Follow-up examination after 3 months of treatment showed the disappearance of nodular change on conventional endoscopy (Fig. 3A), the loss of the tree-like appearance (TLA) on ME-NBI (Fig. 3B), and no residual lymphoma cells on biopsy.
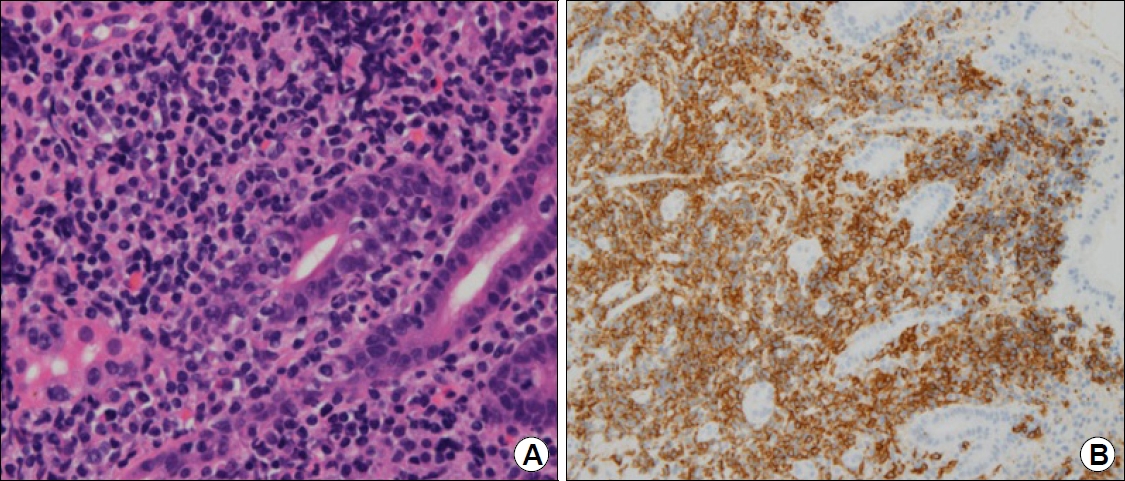
(A) Endoscopic biopsy reveals some clusters of atypical lymphocytes infiltrating the gastric glands (H&E stain, ×400). (B) These lymphocytes are positive for CD20 (CD20 stain, ×400).

(A) Follow-up endoscopy after Helicobacter pylori eradication showing the disappearance of previous nodular changes. (B) Magnifying endoscopy with narrow-band imaging revealing the loss of the tree-like appearance and restoration of pit structures.
Gastric mucosa-associated lymphoid tissue (MALT) lymphoma develops from chronic gastritis, especially as a result of H. pylori infection. Gastric MALT lymphoma can be classified into six types: superficial depressed, multiple erosion, cobblestone, partial fold swelling, submucosal, and discolored. Various macroscopic patterns of gastric MALT lymphoma are often misdiagnosed as gastric ulcer, gastric cancer, or gastritis [1]. Therefore, it is important to include gastric MALT lymphoma initially in the differential diagnoses of lesions identified by conventional endoscopy, particularly when multiple and variable gastric lesions are observed. However, because the lymphoma cells originate from the deep mucosa or submucosa and grow without destroying the foveolar glands, the definitive diagnosis is sometimes difficult even after conducting multiple biopsies.
In the present case, abnormal microvessels resembling long, bare branches from the trunk of a tree, called TLA, were a clue to the diagnosis of gastric MALT lymphoma. A dense infiltration of lymphoma cells is present in the lamina propria and ischemia leads to vascular endothelial growth factor (VEGF) activity [2]. Neovascularization induced by VEGF may be related to the TLA. Because TLA represents the distribution of MALT lymphoma in the stomach, a targeted biopsy under ME-NBI can contribute to the improvement of diagnostic accuracy [2]. This can also reduce the number of biopsies needed. In addition, the disappearance of TLA after H. pylori eradication is useful in judging the successful treatment of TLA-positive gastric MALT lymphoma [3]. However, there are cases of gastric MALT lymphoma without TLA. The frequency of TLA is reported to be 75% in gastric MALT lymphoma [3], and there was no marked difference in the background mucosa between TLA-positive and -negative MALT lymphomas.
In summary, various endoscopic appearances of gastric MALT lymphoma make it difficult to establish a diagnosis by conventional endoscopy alone. The use of ME-NBI can provide a diagnostic aid for gastric MALT lymphoma. Successful treatment results in the disappearance of TLA in TLA-positive MALT lymphoma.
Notes
No potential conflict of interest relevant to this article was reported.
