The Efficacy of a Novel Type of Integrated Knife for Endoscopic Submucosal Dissection in Early Gastric Cancer
Article information
Abstract
Background/Aims
This study aimed to compare the efficacy of a recently developed clear-cut knife with that of widely used knives for endoscopic submucosal dissection (ESD) in early gastric cancer (EGC).
Materials and Methods
Data were collected retrospectively from the National Health Insurance Service Ilsan Hospital between May 2017 and April 2019. The study included 105 patients (108 lesions) with EGC who underwent ESD. Fifty-six EGCs were dissected using an insulation-tipped diathermic knife in combination with a needle knife, while 52 EGCs were dissected using a clear-cut knife, a single device with an integrated needle and an insulated-tipped knife. Oncologic outcomes and technical success were compared between the two groups.
Results
The distribution of the tumor locations, composition of the undifferentiated types of EGCs, and the size of EGCs (14.3±8.0 vs. 16.6±7.3 mm; P=0.130) were not significantly different between the groups. The complete (98.2% vs. 94.2%; P=0.273) and curative (87.5% vs. 88.5%; P=0.878) resection rates were not statistically different. Furthermore, no significant difference between the total procedure times (39±34.1 vs. 35.7±20.1 minutes; P=0.554), areas of the dissected specimen (858.6±406.5 vs. 980.3±393.8 mm2; P=0.119) and dissected areas per minute (32.4±17.9 vs. 35.5±16.2 mm2/min; P=0.345) were noted. Regarding complications, the overall perforation rates (3.6% vs. 4.0%) and bleeding rates (3.6% vs. 8.0%) were not statistically different.
Conclusions
The newly developed clear-cut knife is as useful as the combination of classical knives for ESD in EGC.
INTRODUCTION
Endoscopic submucosal dissection (ESD) has been established as the first treatment option for patients with early gastric cancers (EGC) [1], as long-term clinical outcomes have been favorable [2,3]. Moreover, ESD is a minimally invasive procedure that preserves the majority of the stomach and improves the quality of life of patients [4]. However, ESD remains a technically challenging procedure requiring various accessories for its execution [5]. Most importantly, the selection of the knives for the procedure greatly influences the success of the procedure and its execution time.
The originally devised needle-type knife was first used to precut endoscopic mucosal resections, which are considered an early form of ESD [6]. Tip-cutting knives are still widely used today in the now almost standardized process of ESD. As the process of ESD has been formalized, the tips of the cutting knives have been modified to reduce several technical drawbacks of needle-type knives. By attaching a non-electric ceramic ball to the tip of the cutting knife [7], the perforation rates were decreased. Eventually, an insulation-tipped diathermic knife-2 (IT-2; Olympus, Tokyo, Japan) was introduced [8].
Notably, despite some advantages, a blunt-tipped knife has a problematic disadvantage due to its structure. At the beginning of the circumferential cutting during ESD, the first mucosal incision is essential. However, it is not possible with a blunt-tipped knife alone, and needs the additional use of a needle tip-type knife. Recently, a needle-tipped knife mounted inside a blunt-tipped knife was developed. However, little was known about whether using a combination knife would reduce the ESD procedure time without lowering the technical success rates and oncologic outcomes. In this study, we retrospectively compared the technical success and oncologic outcomes of a newly developed combination knife with those of the IT-2 knife (Olympus).
MATERIALS AND METHODS
1. Patients
From May 2017 to April 2019, 105 patients with a total of 108 lesions among them, were diagnosed with EGC and had undergone ESD at the National Health Insurance Service Ilsan Hospital (Goyang, Korea). Patients whose final diagnosis was compatible with EGC after ESD were included in the study. Patients whose pre-procedural diagnosis was a gastric flat-type adenoma/dysplastic lesion and was confirmed by biopsy as high-grade intraepithelial neoplasia were also included. Where the preprocedural diagnosis was EGC, endoscopic ultrasonography was performed to evaluate whether submucosal invasion was present. Computed tomography was carried out before the ESD to rule out lymph node metastasis.
Discontinuation of drugs which increase bleeding tendencies, such as antiplatelets and anticoagulants, was implemented according to the recommendations of the recent guidelines [9-11] and in consultation with the cardiologist and/or neurologist. These drugs were restarted within 1 week from the day of ESD if post-procedural bleeding did not occur. When patients who should not stop taking such drugs received ESD, the procedure was carried out without stopping the drugs. Proton pump inhibitors were administered intravenously for 3 days and orally for 8 weeks after ESD. This study was approved by the Institutional Review Board (IRB) of the National Health Insurance Service Ilsan Hospital (IRB No: 2019-04-005). As a retrospective observational study, the waiver of the subject's written consent was reported to the IRB and approved.
2. ESD procedures
The endoscopic procedures were performed using a single-channel endoscope (GIF Q260J or GIF-H260Z, Olympus). After endoscopic evaluation of the gastric lesions, markings were placed 5 mm away from the lesion margins by electrocautery (ICC 300; ERBE, Tubingen, Germany) using an argon plasma coagulation (APC) probe. Saline mixed with epinephrine (0.01 mg/mL) and a 0.8% indigo carmine mixture were used for submucosal injection. The severity of submucosal fibrosis was assessed based on the reports of previous a study [12]. The knives to be used for the ESD were selected by the operator according to personal preference and recorded post-procedure. Two different combinations of knives were used for the procedures. All procedures were performed by one operator who had performed over 100 ESD procedures. We compared several indicators for the oncologic outcomes and technical success of ESD between the two groups of knife combinations used for the procedure.
1) IT-2 knife group
The IT-2 knife (KD-611L, Olympus) was used for the main submucosal dissection knife in the control group, since it was one of the most commonly used dissecting blunt-tipped knives in ESD. Circumferential cutting was made in the mucosa by either using the needle-type knife (KD-10Q-1-A, Olympus) by itself or combined with the IT-2 knife. The submucosal layer was dissected mainly using the IT-2 knife. The needle-type knife was used as an assistive device when submucosal fibrosis was severe.
2) H-type clear-cut knife group
The H-type clear-cut knife (FINEMEDIX Ltd., Daegu, Korea) has an insulated tip (O-type knife) except for the center of the tip where the needle is metal (I-type knife), as shown in Fig. 1. Circumferential cutting was made in the mucosa using the I-type cutting knife alone or in combination with the O-type knife. The submucosal layer was dissected mainly with the O-type knife. In addition, the I-type knife was used when submucosal fibrosis was severe.
Endoscopic hemostasis was achieved mainly using hemostatic forceps (FD-410LR; Olympus). However, when thick blood vessels were exposed or bleeding was not properly controlled using hemostatic forceps alone, hemoclips (Olympus) were used.
The VIO generator (VIO 300D; ERBE) was used as an electrosurgical generator. The settings for the IT-2 knife and H-type clear-cut knife were as follows: Endocut Q 4-2-2 for marginal incision and Swift coagulation E2 40 W for submucosal dissection. Soft-mode coagulation was used for hemostasis by hemostatic forceps coagulation (Effect 4 with 80 W).
3. Histopathologic evaluation
The locations of the EGC lesions were divided into three areas along the longitudinal axis of the stomach, from the esophagogastric junction to the pyloric channel: (1) the fundus, cardia, and upper one-third or corpus were categorized as the upper one-third; (2) the lower two-thirds of the corpus and angularis incisure were categorized as the middle third; and (3) the antrum and pylorus were categorized as the lower one-third.
The morphology of the tumors was categorized according to the Japanese Research Society for Gastric Cancer classification system [13] into three main categories based on the predominant type under endoscopic examination as follows: elevated type, flat type, and depressed type [14].
The histological type was classified according to the World Health Organization histological classification [15] and the Lauren classification systems [16]. The specimens were sectioned at 2 mm intervals, centered on the part of the lesion closest to the margin and the site of the deepest invasion. Slides were stained with hematoxylin-eosin. In accordance with the Japanese Classification for gastric carcinomas, the size of the tumor, depth of invasion, tumor involvement of the horizontal and vertical margins, and lymphatic and vascular invasion were evaluated. After measuring both the long and short diameters, the maximum diameter was set as the representative value of the tumor size.
4. Assessment of technical terms and oncologic outcomes [1]
Complete resection was defined as en bloc resected specimens that revealed the lateral and vertical margins to be histologically free of malignant cells without lymphvascular invasion of tumor cells. Resection was considered curative when the final pathologic results met the criteria of ESD, both the absolute indications and the expanded indications (EI), among those who had complete resection. The total procedure time was defined as the interval between the time marking with APC began and the time of retrieval of the resected specimen, after hemostasis and management of other adverse events had been achieved. The dissection time was defined as the interval between the completion of circumferential cutting and trimming and the complete removal of the specimen. We also recorded whether clip devices were used and for what purpose.
5. Statistical analysis
Quantitative variables are described as mean±SD. Comparative analysis between the groups was performed. Numerical variables were compared using the independent t-test or Wilcoxon rank-sum test, and categorical variables were compared using Pearson chi-squared test or Fisher’s exact test, as appropriate. A P-value <0.05 was considered statistically significant. All statistical analysis were performed using SPSS 18.0 for Windows (SPSS, Inc., Chicago, IL, USA).
RESULTS
1. Baseline characteristics of subjects
The baseline characteristics of the patients and of the EGC lesions in the two groups are described in Table 1. The sex ratio and mean age of the patients were not statistically different. Four-fifths of the patients were male and the mean age was about 70.9 years old in the IT-2 knife group and 71.8 years old in the H-type clear-cut knife group. Eight (14.5%) patients in the IT-2 knife group and six (12%) patients in the H-type clear-cut knife group were taking antiplatelets or anticoagulants. A total of five subjects, from both groups, underwent ESD while on medication. In two of the eight patients in the IT-2 knife group, antiplatelets were withdrawn, and in one patient in that group, warfarin/drug of direct oral anticoagulants was withdrawn. Antiplatelets were withdrawn temporarily from one patient in the H-type clear-cut knife group.
Morphologically depressed EGCs were more frequently observed in the control group than in the H-type clearcut knife group (62.5% vs. 29.6%; P=0.002). In both groups, approximately 66% of the tumors were located in the lower-third, and approximately 33% of the EGCs were located in the mid-third. In both groups, approximately 25% of the lesions were accompanied by subepithelial fibrosis.
Approximately 96% of the tumors were differentiated-type EGCs, which consisted of well and moderately differentiated adenocarcinomas. Well-differentiated adenocarcinoma was more frequently reported in the IT-2 knife group (82.1% vs. 55.8%; P=0.008) than in H-type clear-cut knife group.
The mean size of the tumors was less than 20 mm in both groups, and there was no statistical difference between the groups (14.3 vs. 16.6 mm, respectively; P=0.130). Of the lesions, 85% were confined to the epithelial layer. Eight (16.1%) lesions in the IT-2 knife group and eight (15.4%) lesions in the H-type clear-cut knife group were reported as submucosal invasion pathologically.
2. The oncologic and technical outcomes of ESD
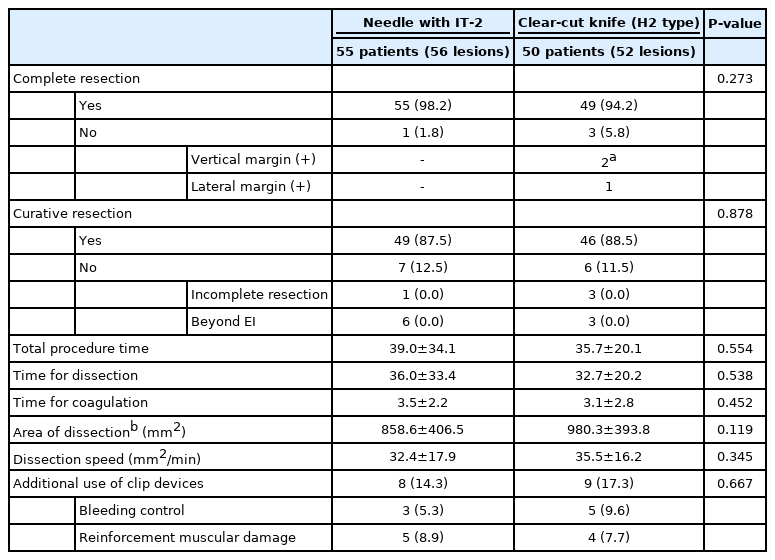
The oncologic outcomes and technical success of ESD are shown in Table 2. Complete resection rates were not different between the groups (P=0.273). Overt perforation occurred in one patient in the IT-2 knife group; surgical resection of the tumor was performed, and the procedure was classified as an incomplete resection. Two lesions had positive vertical margins and one lesion showed lateral margin positivity in the H-type clear-cut knife group. The curative resection rates were also not different between the groups (P=0.878). Seven (12.5%) lesions were classified as non-curative resections in the control group, and six (11.5%) lesions were beyond the EI of ESD. In the H-type clear-cut knife group, six lesions were classified as non-curative resections and three lesions were beyond the EI.
The total procedure time (39±34.1 vs. 35.7±20.1 minutes; P=0.554), time for dissection (36±33.4 vs. 32.7±20.2 minutes; P=0.538) and time for coagulation (3.5±2.2 vs. 3.1±2.8 minutes; P=0.452) were not statistically different between the two groups. The area of dissection and dissection speed did not make any difference.
3. The complication of ESD
The overall complication rate was 25.5% in the IT-2 knife group and 22.0% among patients in the clear-cut knife group (Table 3).
Overall perforation rates were not different between the groups. Overt perforation occurred in one patient (1.8%) in the IT-2 knife group, and the EGC was surgically resected by gastrectomy. Minute perforation, which is defined by minimal free air in simple X-rays without symptoms or signs of systemic inflammatory response syndrome (SIRS) and abdominal pain, occurred in one patient in the IT-2 knife group and two patients in the H-type clear-cut knife group. Cases of minute perforation were treated without any comorbidity.
Two cases (3.6%) of post-procedural bleeding occurred in the IT-2 knife group, and four (8%) patients suffered from bleeding in the H-type clear-cut knife group. Two of the four patients experienced acute bleeding, which occurred within 48 hours after ESD.
The most frequently observed complications were post-procedural fever, defined as a fever higher than 38 degrees celsius [17] without any other signs of SIRS, and abdominal pain. Ten (18.2%) patients in the IT-2 knife group and five (10%) patients in the H-type clear-cut knife group showed symptoms of post-procedural fever (P=0.231). Pneumonia did not occur.
DISCUSSION
To the best of our knowledge, this is the first study comparing the efficacy of a novel type of integrated knife with the conventional combination of knives. Our results showed that the H-type clear-cut knife achieve equally effective oncologic outcomes and technical success without increasing the risk of complications as the conventional combination of knives. In terms of the expenses, using the novel two-in-one knife system demonstrates a cost advantage. Therefore, the H-type clear-cut knife could be an effective alternative when choosing the appropriate knife for ESD in EGC.
ESD has enabled larger mucosal resections to be technically possible with higher rates of en bloc, complete and curative resections compared to mucosal resection in EGCs [18]. As such, ESD has been adopted as a treatment of choice in EGCs. However, obstacles associated with ESD are that it is generally time-consuming and technically difficult. Higher risks of complications such as post-procedural bleeding and perforation are sometimes inevitable [19]. To overcome the technical hurdles, various auxiliary devices for ESD have been developed [20]. Certainly, the importance of endoscopic knives is beyond doubt [8,21,22]. Currently, there are few studies directly comparing the outcomes of the various ESD knives.
The IT-2 knife has been structurally improved through integration of the diathermic tip of the original, the IT type, and the triangle-tipped knife in order to overcome several technical drawbacks of the original IT knife [8]. Recently, an effective new type of hybrid knife with an integrated water-jet system and needle electrode has been developed [22]. The newly developed H-type clear-cut knife combines a needle electrode within the diathermic knife, combining the advantages of both knives. Importantly, the additional benefits of the H-type clear-cut knife are minimizing the number of device changes and maintaining the safe cutting plane within the submucosal layer by the alternate use of two knives. Considering ESD is a time-consuming and technically difficult procedure, simplifying the process of ESD could contribute to advanced operability. Thus, the feasibility of this new device has been tested in this study.
We have shown here that the complete and curative resection rates and the major parameters used to evaluate the short-term outcomes of endoscopic treatment were acceptable, as compared to the reported outcomes of ESD for EGC in previous studies [23,24]. When only the cases whose final pathologic reports of EGCs meet the absolute indication (AI) or EI, excluding lesions that do not meet either the AI or the EI, were analyzed, the complete resection rates were 98.2% (55/56 lesions) in the IT-2 knife group and 94.2% (49/52 lesions) in the H-type clear-cut knife group, and the curative resection rates increased to 98% (49/50 lesions) in the IT-2 knife group and 93.9% (46/49 lesions) in the H-type clear-cut knife group.
In two previous studies, the median procedure time for the IT-2 knife was 60.5 minutes (range: 44 to 86.3; mean: 79.3) [22] and 35 minutes (range: 7 to 300; mean: 48) [8]. In another study by Huang et al. [22] using a hybrid knife resulted in reducing the procedure time to 43 minutes (range: 27 to 60) compared to the total procedure time of 60.5 minutes (range: 44.0 to 86.3) when using an IT-2 knife. This was statistically significant. The mean procedure time using the H-type clear-cut knife was 35.7 minutes, although the expected reduction in procedure time could not be confirmed. This is probably because the needle-type knife is used only at the beginning of the precut. Therefore, the insulated-tipped knife is used for most of the procedure, in the second half of precutting and during submucosal dissection. Furthermore, it is possible that the needle-type knife was used only when the approach angle of the IT knife was not appropriate or when it was difficult to cut with an IT knife due to submucosal fibrosis or burning of the submucosal tissue by electric cauterization. Since those situations do not occur frequently during submucosal dissections, there are not many situations in which the two knives should be used alternately during submucosal dissection.
The overall rate of complications demonstrated in this study is somewhat higher than in previous studies [25,26]. This is because post-procedural fever was included as a complication in this study. Post-procedural fever has been reported in 25% of patients after ESD [27]. The complication rate without fever is similar to that of previous reports. No significant difference in overall complications between the two groups in our study was found. Post-procedural fever occurred in about 15% of patients, but there were no additional symptoms of abdominal pain or SIRS. Postprocedural fever is a well-known symptom that occurs after endoscopic removal of colonic neoplasms, referred to as post-polypectomy syndrome. Little has been reported about this type of complication, which is presumably caused by transmural burns, especially in ESD of EGCs. A previous study by Nakanishi et al. [27] suggested that post-procedural fever in ESD of EGC might be associated with the diameter of the dissected lesion. In the corresponding study, 19.5% of patients experienced post-ESD pyrexia, and the average resection diameter was 35 mm.
In this study, the dissected area was presented instead of the diameter of the resected lesion. The average diameter of the resected piece was 37.4 mm, 36.0 mm in the IT-2 group and 38.1 mm in the clear-cut knife group. Since there was no significant difference in the diameter of the resected tissue from the previous study, post-ESD pyrexia may have occurred at a similar rate in this study.
Taken together, these results suggest that the use of the newly developed knife does not yield inferior results in the technical aspects and oncologic outcomes of ESD of EGCs. Moreover, the newly developed knife does not increase the incidence of complications. Since there was no difference in the medical index outcomes of ESD, these results can help an operator choose which knife to use. We did not describe the expenditure for the procedure, nor did we compare the cost of the knives in this study. However, considering the medical expense of using multiple instruments, two-in-one systems might have a cogent advantage, especially when it is difficult to use multiple tools simultaneously due to insurance coverage issues, as is the case in South Korea.
The limitations of this study are as follows. Firstly, this study is a retrospective study conducted by single operator in a single institution. This restricts the sample size and diversity in operators. Moreover, it is difficult to completely rule out the possibility that the shape of the lesion influenced the operator's choice. Secondly, the procedure time and technical success rate may depend on the individual ability of the operator. In order to decrease operator bias and to achieve scientific confidence, a large number of randomized controlled trials in multicenter settings is required.
In conclusion, the H-type clear-cut knife and the IT-2 knife were equally effective and safe for successful ESD in EGCs. The H-type clear-cut knife appears to offer an advantage over the conventional knives with its two-in-one system, reducing the expenditure for instruments.
Notes
No potential conflict of interest relevant to this article was reported.