Dysphagia Secondary to Esophageal Compression in a Patient with Decompensated Heart Failure
Article information
Abstract
Cardiogenic dysphagia is a rare type of esophageal dysphagia caused by external compression of the esophagus by an enlarged left atrium. Long-term comparisons between the degree of cardiogenic dysphagia and heart failure have not been reported due to its low incidence. We hereby report the case of a 74-year-old woman with valvular heart disease, suspected of having oropharyngeal dysphagia following a recent intracerebral hemorrhage, who performed a swallowing function test. Videofluoroscopic swallowing study (VFSS) revealed a supraglottic penetration, confirming the oropharyngeal dysphagia. Furthermore, post-VFSS chest radiograph revealed esophageal residual barium, suggestive of reduced esophageal food transition secondary to external compression, at the level of the T6 vertebral body. Chest computed tomography showed mid-esophageal compression caused by left atrial enlargement. She had pulmonary edema which was managed with diuretics. Post-VFSS chest radiographs also revealed a direct association between the diameter of the esophageal barium residue and body weight. A reduction in body weight led to the resolution of the barium residue and vice versa. Development of cardiac dysphagia may be one of the signs of acute exacerbation of heart failure.
INTRODUCTION
Esophageal dysphagia is often caused by factors such as intraesophageal masses or strictures, external compression, or motility disorders. However, in rare cases, external compression of the esophagus involves enlarged or anomalous cardiovascular structures, such as an enlarged left atrium (LA) (dysphagia megalatriensis) [1]; dilated, tortuous, aneurysmal thoracic aorta (dysphagia aortica) [2], or aberrant subclavian artery (dysphagia lusoria). Furthermore, LA enlargement, massive four-chamber cardiac dilatation [3], or pericardial effusion [4] can cause esophageal compression often referred to as cardiogenic dysphagia [5]. Cardiogenic dysphagia, dysphagia aortica, and dysphagia lusoria are collectively called cardiovascular dysphagia [5].
Esophageal dysphagia has been suggested as an early sign of cardiac decompensation in patients with heart failure [6]. Improvements in cardiogenic dysphagia were reported after volume control with diuretics [5-7]. Contrarily, ranitidine treatment was unable to ameliorate the symptoms of dysphagia [3]. The severity of cardiogenic dysphagia does not appear to be constant and varies with the patient’s body volume. However, there are no reports consecutively comparing changes in body weight and severity of cardiogenic dysphagia.
Herein, the authors report a case of cardiogenic dysphagia with temporal changes showing both amelioration and exacerbation of esophageal dysphagia with heart failure control.
CASE REPORT
A 74-year-old female patient with moderate mitral valve stenosis, aortic valve stenosis, and a history of cerebral infarction complained of headache and a decreased consciousness. A diagnosis of intracerebral hemorrhage (ICH) in the right temporal lobe was confirmed. Craniotomy and ICH removal surgery was performed; however, the patient continued to experience confusion. Nasogastric tube feeding was initiated.
Four weeks later, the patient was transferred to the Department of Rehabilitation Medicine. The patient remained on nasogastric tube feeding and a videofluoroscopic swallowing study (VFSS) was performed to evaluate swallowing function. VFSS results revealed supraglottic penetration (Fig. 1), indicating oropharyngeal dysphagia; hence, thickened fluids were recommended. A chest radiograph was obtained immediately after VFSS that confirmed residual barium in the esophagus. The diameter of the barium residue was greater than 1 cm and it was located at level of the T6 vertebral body. Additionally, the shape of the barium residue was indicative of external compression on the esophagus. Chest radiography also revealed features of cardiomegaly with a widened carina angle suggestive of LA enlargement [8]. Echocardiographic assessment showed considerable LA enlargement with an LA volume index of 104.84 mL/m2. Chest CT demonstrated esophageal compression at the level of the T7 vertebral body by the enlarged LA. Serum brain natriuretic peptide (BNP) level was 535 pg/mL. Esophagogastroduodenoscopy revealed a subepithelial lesion covered by normal-appearing mucosa in the midesophagus suggestive of external compression. High-resolution esophageal manometry showed normal esophageal motility. However, in the resting state, a high-pressure zone (14~20 mmHg compared to 5 mmHg in other parts of the esophagus) was noted at the mid to lower esophagus (29~39 cm from the nares) (Fig. 2).

Supraglottic penetration (arrow) while swallowing 5 mL volumes of thin fluid (A) and thickened fluid (B) during videofluoroscopic swallowing study #1.

Post-videofluoroscopic swallowing study (VFSS) #1 chest radiograph, chest CT, esophagogastroduodenoscopy, esophageal manometry findings, and VFSS #4 chest radiograph. (A) Post-VFSS chest radiograph showing an esophageal residual barium of 1.46 cm diameter ending at the T6 vertebral level. An enlargement of the cardiac silhouette and an increase of the carinal angle (white dotted line) to 102º are also noted. (B, C) Chest CT axial (B) and sagittal (C) images showing the enlarged left atrium (LA) with its largest diameter at the T7 body level. The esophagus and nasogastric tube inside the esophagus (white arrow) are located adjacent to the posterior side of the LA. (D) Esophagogastroduodenoscopy image showing a subepithelial lesion (white arrow) indicating external compression. (E, F) High-resolution esophageal manometry studies showing normal esophageal motility (E) and a resting high-pressure zone at the mid to the lower esophagus (F, black arrow). (G) Carinal angle (white dotted line) was 77º during VFSS #4. There was no esophageal residual barium.
After the initial VFSS, the patient was placed on a dysphagia diet consisting of thick rice gruel, minced side dishes, and thickened water. The patient was also instructed to maintain an upright posture for at least 30 minutes after eating. The patient was delirious with no complaints of food sticking in the chest or regurgitation when consuming the dysphagia diet. Preload reduction was achieved through diuretic treatment (furosemide 20 mg p.o. q.d.).
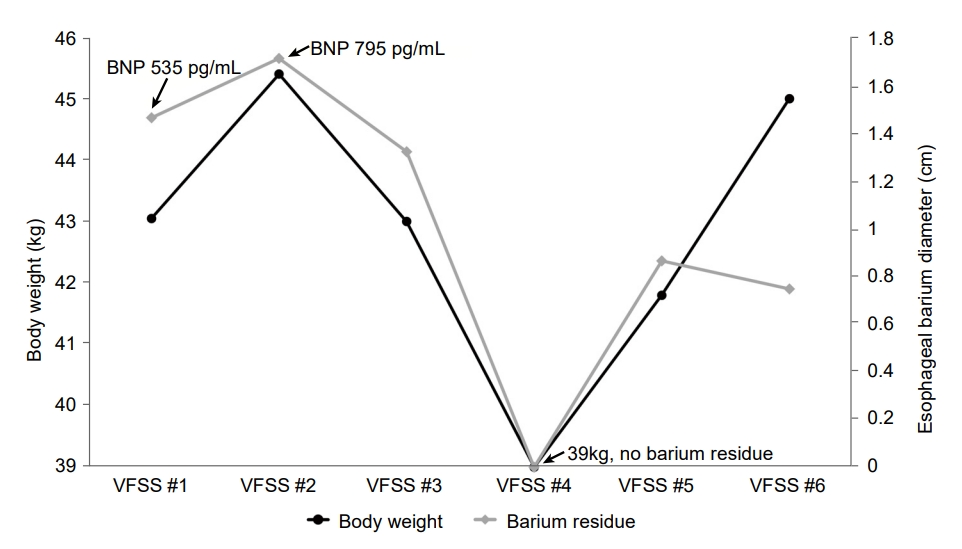
Over the course follow-up, a total of five additional VFSS tests were performed; twice during hospitalization at a 2-week interval and thrice at a 3-month interval after discharge. A close relationship between changes in body weight and the size of esophageal barium residue was observed in the post-VFSS chest radiograph. In the second VFSS, the esophageal barium residue was slightly increased, and the BNP level was 795 pg/mL. The patient developed atrial fibrillation and tachycardia; hence, digoxin 0.25 mg p.o. q.d. was prescribed. During the third VFSS, a decrease in body weight showed a concurrent reduction in esophageal barium residues. After discharge, the dose of furosemide was increased to 40 mg p.o. q.d. and spironolactone (12.5 mg p.o. q.d.) was included in the patient’s medication regimen in the outpatient clinic. Upon reducing weight from 43.1 kg (VFSS #1) to 39 kg (VFSS #4), the post-VFSS chest radiograph showed reduced carinal angle from 102º to 77º and the absence of esophageal barium residue (Fig. 2G, Fig. 3). At the time of VFSS #6, the body weight increased to 45 kg, while esophageal barium was similar with that of VFSS #5 with carinal angle 71º. At the timing of last VFSS, the 6th, echocardiography was performed, and LA volume index was reduced from the initial 104.84 to 81.75 mL/m2.
DISCUSSION
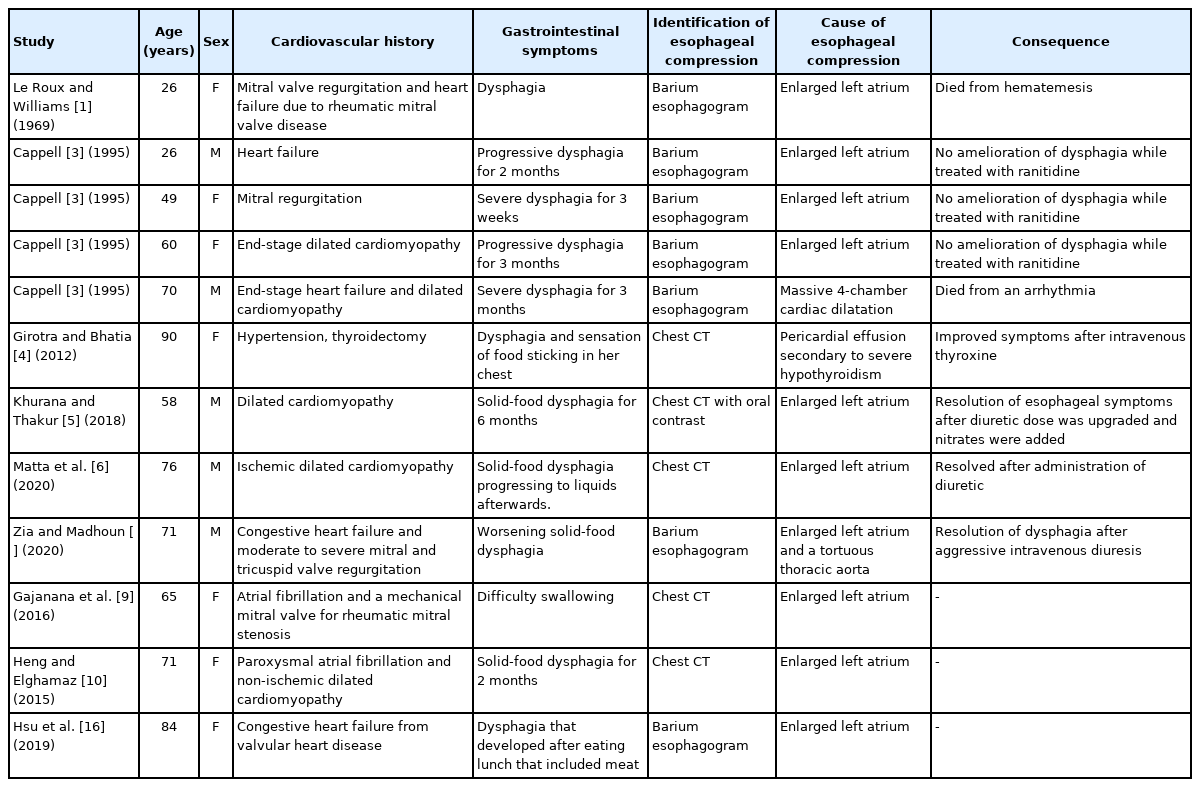
Reports on cardiogenic dysphagia secondary to cardiomegaly are limited. The esophagus passes through the mediastinum adjacent to the heart and great vessels. Esophageal dysphagia may be caused by compression of the esophagus due to an enlarged LA [6], tortuous thoracic aorta, or aberrant right subclavian artery. Cardiogenic dysphagia mostly occurs in patients with heart failure and is associated with valvular heart disease [1,9], dilated cardiomyopathy [5,10], or ischemic cardiomyopathy (Table 1) [2,6]. Rheumatic fever, which has been rarely reported in the past, is the major cause of the compression of the enlarged LA [1]. With the reduction of the incidence of rheumatic fever, reports of esophageal compression due to rheumatic valvular disease have declined. However, in older patients with chronic cardiovascular disease, esophageal compression due to an enlarged LA or thoracic aorta has been reported [2]. Additionally, exacerbation of cardiovascular dysphagia is associated with volume overload. With this, the symptoms of dysphagia improve with adequate control of volume overload.
Co-occurrence of cardiogenic dysphagia and oropharyngeal dysphagia has not been reported. Herein, the authors presented a case of cardiogenic dysphagia with concomitant oropharyngeal dysphagia. Esophageal dysphagia is often caused by disorders distinct from those causing oropharyngeal dysphagia. Intraesophageal masses or strictures, external pressure, and motility disorders are the common etiologies of esophageal dysphagia. In contrast, oropharyngeal dysphagia is often caused by disruption of neural control or structural changes resulting from conditions such as stroke, oropharyngeal cancer, and peripheral nerve disease.
However, some diseases such as Parkinson’s disease are associated with oropharyngeal and esophageal dysphagia [11]. This may be due to a common risk factor associated with oropharyngeal and esophageal dysphagia. Furthermore, the incidence of stroke is linked with cardiovascular risk, especially among patients with chronic heart disease. The present study reported the development of oropharyngeal dysphagia after having an intracerebral hemorrhage in a patient with known valvular heart disease.
In this case, cardiogenic dysphagia was an incidental finding while the patient was primarily evaluated for oropharyngeal dysphagia. VFSS, the modified barium swallow, is a widely used standard assessment tool for oropharyngeal dysphagia. Several VFSS laboratories evaluate chest radiograph after VFSS to detect endotracheal barium, which is evidence of endotracheal aspiration [12]. Authors have previously suggested that residual esophageal barium in a chest radiograph routinely taken immediately after VFSS could indicate esophageal dysphagia [13]. Cardiogenic dysphagia can be suspected if there is a history of previous heart disease and if evidence of cardiomegaly is present on the chest radiograph. If esophageal dysphagia is suspected, assessment for abnormalities may be done through esophagogastroduodenoscopy and manometry. Contrarily, chest computed tomography and echocardiography must be considered to identify the enlarged LA for patients in whom cardiogenic dysphagia is suspected.
Severe LA enlargement is often assessed by LA anterior-posterior dimension ≥52 mm in male and ≥47 mm in female [14]. The correlation between LA diameter and angle of tracheal bifurcation was reported [8]. Carinal angle larger than 90º or more is accepted as indicator of LA enlargement. In this case, we showed the presence and improvements of esophageal dysphagia were related with body weight changes and carinal angle. Moreover, the location of external esophageal compression was observed in the subcarinal area, where the enlarged LA locates. From the initial VFSS to the 5th VFSS, size of esophageal barium residue and body weight changed showed close relationship. In the 6th VFSS, the body weight further increased but the size of residue was not changed compared to the 5th test. We assumed that the increased body weight was not entirely due to the aggravation of cardiac function because the LA volume index decreased. Comparing the VFSS #1 with the VFSS #6, although the body weight slightly increased, the measured LA volume index decreased. The lowest carinal angle measured was at VFSS #6. Comparing carinal angle with LA volume index at VFSS #6 and VFSS #1, carinal angle reflects LA volume. However, considering VFSS #4, the carinal angle may have been measured too small at VFSS #6. Because the carina is a dynamic structure, if the chest radiograph was taken in the patient's maximal inspiratory state, the carinal angle may have been narrowly measured compared to those in expiratory state [15]. The degree of inspiration might not be equally controlled for each chest radiograph because of difference in cooperation. Despite these limitations, chest radiography is readily obtainable, inexpensive, not technically difficult, and is widely performed. Tracheal bifurcation angle is easy to measure as the subcarinal angle. The body weight and carinal angle can be used as a monitor for LA enlargement.
In summary, cardiogenic dysphagia was identified in a 74-year-old female patient with valvular heart disease. After management of exacerbated heart failure, body weight and carinal angle were reduced, and improvement of cardiogenic dysphagia was observed. Swallowing difficulty that occurs in patients with heart failure may be associated with external esophageal compression by LA enlargement and one of the signs of acute exacerbation of heart failure. Along with conventional evaluations for dysphagia, such as VFSS, esophagogastroduodenoscopy, and esophageal manometry, chest CT could be useful assessing the cause and location of cardiogenic dysphagia.
Notes
No potential conflict of interest relevant to this article was reported.
Acknowledgements
This study was presented at the 2021 Korean Dysphagia Society Fall Academic Conference.
This case report was approved by the Institutional Review Board of the Kangwon National University Hospital (IRB No. KNUH-2021-07-029).