Usefulness of the Kyoto Classification Score for Prediction of Current Helicobacter pylori Infection
Article information
Abstract
Background/Aims:
Based on the Kyoto classification of gastritis, mucosal atrophy, endoscopic intestinal metaplasia, fold enlargement, nodularity, and diffuse redness may be associated with gastric cancer and Helicobacter pylori (H. pylori) infection. In this study, we investigated the association between Kyoto scores based on the aforementioned five variables and current H. pylori infection.
Materials and Methods
We reviewed medical records of consecutive patients who underwent endoscopic biopsies between January and June 2019. The study included 687 patients (370 and 317 patients with H. pylori-negative and -positive results, respectively). The Kyoto score was evaluated by the endoscopist who performed the test and was reconfirmed by another endoscopist. The total Kyoto score was analyzed using a receiver operating characteristic (ROC) curve for each score from 0 to 8. Multivariate analysis was used to determine the variables associated with H. pylori infection.
Results:
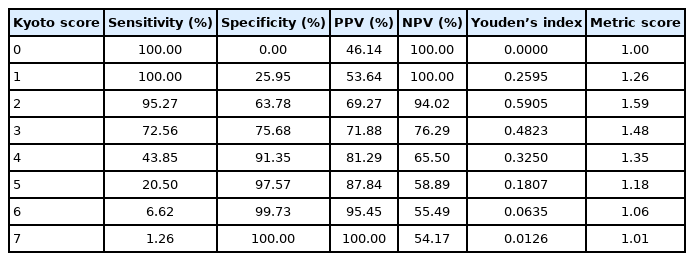
The maximum value of the Youden index (which reflects the ideal cut-off score of the Kyoto score on the ROC curve) was a Kyoto score of 2 points (Youden index 0.5905). Nodularity (OR 24.69, 95% CI 8.57~71.16, P<0.001) and diffuse redness (1 point: OR 18.29, 95% CI 10.29~32.52, P<0.001 and 2 points: OR 30.82, 95% CI 14.07~67.52, P<0.001) showed the highest OR on multivariate analysis.
Conclusions:
A Kyoto classification cut-off score of 2 points was suggestive of H. pylori infection, and mucosal nodularity and diffuse redness were most significantly associated with the risk of infection.
INTRODUCTION
Helicobacter pylori (H. pylori) was identified in the 1980s, with an overall prevalence of more than 50% worldwide [1,2]. The World Health Organization and the International Agency for Research on Cancer have categorized H. pylori as a carcinogen [3,4]. Therefore, appropriate detection of H. pylori infection followed by its eradication is important [5,6].
In Sydney classification, the gastritis is diagnosis by histologically and endoscopically [7]. This is too complicated to be used well in actual clinical field. In 2013, the Japan Gastroenterological Endoscopy Society developed the Kyoto classification, which can assess H. pylori infection status and predict gastric cancer risk [8]. In the Kyoto classification, endoscopic findings related to H. pylori and drug-induced gastric mucosal damage were expressed on 19 parameters. Unlike the conventional method of detecting H. pylori infection using invasive and non-invasive methods [9], the Kyoto classification of gastritis has made it possible to predict H. pylori infection only by endoscopic inspection. The Kyoto classification of gastritis consists of five endoscopic findings related to gastric cancer: extent of atrophic mucosal change, intestinal metaplasia (IM), enlargement of gastric folds, nodularity, and diffuse redness, which are scored from 0 to 8 [4,10]. A Japanese study found that a score of 2 or higher per the Kyoto classification strongly suggested a current H. pylori infection and a score of 4 or higher indicated a high risk of gastric cancer [4]. In addition, certain endoscopic findings, such as nodular pattern in the antrum or diffuse redness in the stomach body suggest H. pylori infection [5,6]. Other findings were associated with future stomach cancer risk [4,11-14].
In Korea, adults aged 40 or older are encouraged to undergo gastroscopy once every 2 years for early diagnosis of gastric cancer. It is important for endoscopists to not only diagnose gastric cancer early but also to actively test for H. pylori infection, and treat it if infection is confirmed, for the prevention of gastric cancer. Thus, the five parameters of the Kyoto classification of gastritis can be used as the basis for simply determining whether there is H. pylori infection in the clinical field. There are few studies in Korea on the Kyoto classification of gastritis and the predictability of H. pylori infection. Therefore, in this study, we investigated the association between scores based on five parameters of the Kyoto gastritis classification, and the presence of H. pylori infection. In addition, we tried to find out which of the five parameters could reflect the current H. pylori infection in Korea.
MATERIALS AND METHODS
1. Patients
We reviewed of consecutive patients’ medical records who did esophagogastroduodenoscopy and special diagnostic procedures for H. pylori detection from January 2019 to June 2019 in the Eunpyeong St. Mary’s Hospital outpatient center of gastroenterology and screening endoscopy center. The special diagnostic procedures for H. pylori detection included gastric mucosal biopsy with the Warthin-Starry stain or a molecular method using dual priming oligonucleotide-polymerase chain reaction (DPOPCR) U-TOP HPy-ClaR Detection Kit (Seasun Biomaterials, Daejeon, Korea).
We included patients aged >19 years who were underwent gastric mucosal biopsy with the Warthin-Starry stain or a molecular method using dual priming oligonucleotide-polymerase chain reaction. We excluded patients aged >80 years, those with a prior history of malignancy of the gastrointestinal (GI) tract, or pancreaticobiliary tract; those who were pregnant or breastfeeding; and those with a history of major abdominal surgery (excluding simple closure of uncomplicated GI tract perforation, appendectomy, cholecystectomy, hysterectomy, or endoscopic mucosal resection/endoscopic submucosal dissection to treat stomach neoplasms). Patients with active gastric ulcers were excluded. This study was approved by the Ethical Review Committee of the Catholic Medical Center of Korea and registered to the Institutional Review Board (PC20RISI0136).
We collected the medical records of 870 patients and excluded 183 patients (12 had malignancy in GI tract, 36 had active gastric ulcers, eight were aged under 20, 48 were aged over 80, 20 had poor endoscopic images, five had undergone major operations on the GI tract, and 54 had some missing medical records). A total of 687 patients were enrolled, of which 370 were H. pylori-negative and 317 were positive (Fig. 1).
2. Kyoto scoring system
The Kyoto score consists of five endoscopic findings, scored from 0 to 8 points. Atrophic mucosal change was evaluated using the Kimura and Takemoto classification system [15] and scored from 0 to 2 points. C-0 and C-I correspond to 0 points, C-II and C-III are scored as 1 point, atrophic changes further than C-III (from O-I to O-III) are scored as 2 points. IM of the gastric mucosa is often observed as gray to white colored with slight mucosal elevation [4]. IM can be easily detected by image-enhanced endoscopy. However, in evaluating IM using the Kyoto classification, image-enhanced endoscopy is not used [4,16]. If IM is absent, it is scored as 0; if IM exists only at the antrum, it is scored as 1, and if IM exists further in the gastric body, it is scored as 2 points. Moreover, if a gastric fold exceeding 5 mm exists, 1 point is added. Nodularity refers to “chicken skin-like” nodular gastric mucosal change, which corresponds to 1 point in the Kyoto classification score. Finally, diffuse redness, which is the overall reddish mucosal change due to superficial gastritis, is mainly observed in the non-atrophic mucosa [4]. In scoring diffuse redness, regular arrangement of collecting venule (RAC), which is a normal mucosal appearance of the stomach body, observed as a starfish-like finding, could be helpful [4,11]. Mild diffuse redness or diffuse redness showed with RAC is scored as 1, while severe diffuse redness without RAC (destroyed collecting venules) is scored as 2. In our endoscopic center, each endoscopist recorded the total Kyoto score and its five variables in the endoscopic report from 2019. Endoscopy was performed by two endoscopists (Oh JH, Lim CH) who were active as gastroenterologists with 15 and 11 years of experience at the time of the examination. They described the Kyoto score on the endoscopic report. The corresponding author educated the first author on the Kyoto scores through the papers in references 2 and 4. The author and corresponding author then reviewed all endoscopic pictures and re-evaluated them. When differences were found between the original inspector and the authors, both authors reviewed the photos and assigned the final score. In the case of omission of recordings on the Kyoto score, the first author and corresponding author reviewed the endoscopic photo and completed the Kyoto score.
3. Collecting medical records
The authors reviewed the medical records of enrolled patients, especially their age, sex, height, body weight, body mass index (BMI), smoking history, alcohol consumption history, past medical history (hypertension, diabetes mellitus, chronic kidney disease, chronic liver parenchymal disease, prior history of major surgery), and history of H. pylori eradication. H. pylori infection status and histologic diagnosis were reviewed.
4. Statistical analysis
We evaluated differences in age, sex, height, weight, BMI, alcohol consumption, smoking history, and comorbidities between the H. pylori-positive and H. pylori-negative patients. We performed a receiver operating characteristic (ROC) curve analysis to select the optimal sensitivity and specificity for diagnosing H. pylori and set the cutoff score for the Kyoto classification of gastritis. We performed a multivariate logistic regression analysis to evaluate which parameter of the Kyoto classification of gastritis would likely to predict H. pylori infection. R version 4.0.0 (R Foundation for Statistical Computing, Vienna, Austria) was used.
RESULTS
1. Patient enrollment and baseline characteristics
Table 1 is patients’ baseline characteristics. There were statistical differences in age and sex between the H. pylori-negative and positive groups. The mean age of the H. pylori-negative group was 62.9±11.4 and that of the positive group was 58.8±12.2 (P=0.000). In the H. pylori-negative group, there were 179 males (48.4%) and 191 females (51.6%); in the H. pylori-positive group, there were 124 males (39.1%) and 193 females (60.9%) (P=0.018). However, there were no statistical differences in the BMI (P=0.988) and history of comorbidities (P=0.467) between the H. pylori-negative and positive groups.
2. ROC curves according to the Kyoto score
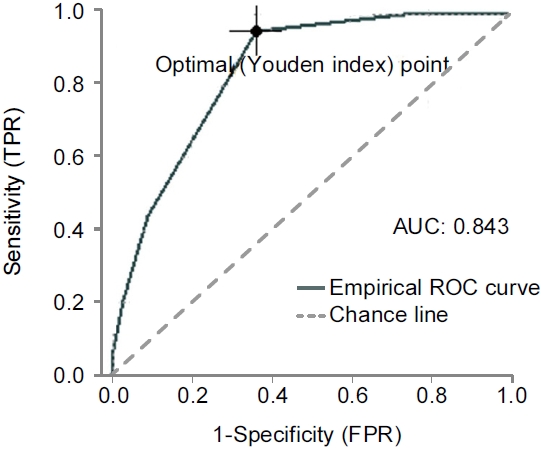
Youden’s index (the value of sensitivity+specificity-1), which reflects the best cut-off score for suggesting H. pylori infection, was a Kyoto score of 2 points (Youden’s index was 0.5905). Table 2 shows Youden’s index and the metric score of the ROC curve for each Kyoto score in this study. Therefore, the ideal cut-off score for predicting H. pylori infection using the Kyoto classification score was also 2 points in South Korea (Fig. 2).
3. Multivariate regression analysis of parameters
To examine the power of each parameter of the Kyoto classification for predicting H. pylori infection, we performed a multivariate regression analysis (Table 3). The OR of 1 point for atrophy was 8.63 (95% CI 4.42~16.86, P<0.001). For 2 points in atrophy, the OR was 16.84 (95% CI 7.69~36.88, P<0.001). The OR of 1 point in endoscopic IM was 2.03 (95% CI 1.22~3.36, P=0.001). The OR of 2 points in endoscopic IM was 1.33 (95% CI 0.73~2.40, P=0.347). The OR of enlarged fold was 1.33 (95% CI 0.34~5.18, P=0.683). The OR of nodularity was 24.69 (95% CI 8.57~71.16, P<0.001). The OR of mild diffuse redness was 18.29 (95% CI 10.29~32.52, P<0.001). The OR of severe diffuse redness was 30.82 (95% CI 14.07~67.52, P<0.001). In this analysis, nodular mucosal change and diffuse redness showed the two largest ORs, which was consistent with the findings of the original study.
DISCUSSION
In this study, we verified that the cut-off score of the Kyoto score for suggesting H. pylori infection was 2 points in Korea. In addition, the most important factors in the scoring system for predicting H. pylori infection were mucosal nodularity and diffuse redness. The Kyoto classification of gastritis suggested by Japan could be a novel and intuitive method for predicting H. pylori infection and gastric cancer risk [4]. There were few data on the Kyoto classification score in Korea. In a study using the endoscopic Kyoto scoring system, sensitivity, specificity, and AUC were found to be 91.0%, 82.1%, and 0.88%, respectively, when the diffuse redness was a one-point cutoff value [17]. Since diffuse redness is one of the important parameters that can predict H. pylori infection, it was confirmed in the present study that if diffuse redness was seen in endoscopic findings, there was a high probability of H. pylori infection. In a previous study in Japan, it was found that a Kyoto score of 2 or more indicated a current infection with H. pylori [4]. The result of our study was similar with this, in which the maximum Youden’s index on the ROC curve was a Kyoto score of 2 points. Recently, there has been a study on the usefulness of the Kyoto classification of gastritis, examining the diagnostic performance of 16 endoscopic findings of the Kyoto classification. Patients were classified into three groups according to the serum H. pylori IgG titer: non-infection, past-infection, and current infection [18], in which the two most important parameters predicting H. pylori infection were mucosal atrophy and diffuse redness, whereas it was nodularity and diffuse redness in the present study. In the Japanese study, the status of H. pylori infection was categorized using a serological test; however, we judged existence of H. pylori according to the biopsy results.
This study had some limitations. First, there may be interindividual variations. To correct this, all endoscopic pictures were reviewed. The Kyoto classification of gastritis was recorded by the original endoscopist who inspected it; thereafter, the author and corresponding author reviewed all endoscopic pictures and re-evaluated it. When differences were found, both authors reviewed the photos together and assigned the final score. Second, our research was performed in one clinical center, and selection bias may have occurred. Third, as shown in Table 1, there were statistical differences in sex and age between the H. pylori-positive and negative groups. However, to overcome these limitations with respect to various biases, we collected consecutive patient data from January to June 2019 instead of extracting the data of H. pylori-positive and negative patients according to the required sample size.
The main strength of this study that it proposes a cut-off value of the Kyoto score that can predict H. pylori infection by comparing the Kyoto scores of patients who had never been infected, with the Kyoto scores of currently infected patients through invasive methods such as biopsy with the Warthin-Starry stain, DPO-PCR or rapid urease test. Thus, if there is diffuse redness in the body or nodularity in the antrum observed via gastroscopy, the presence of H. pylori should be verified through invasive methods. In fact, in clinical practice, it is important to screen for gastric cancer through gastroscopy, and furthermore, it is expected that gastric cancer can be prevented by identifying and treating H. pylori infection that causes gastric cancer through the Kyoto classification of gastritis.
Taken together, unlike OLGA and OLGIM systems, the Kyoto scoring system does not require invasive methods, so it can be easily used in actual clinical practice. In a real clinical field, it is not possible to describe all variables from the Kyoto classification of gastritis, therefore, recording values for five variables related to Kyoto score will help to manage the patients. The results of the present study showed that the Kyoto score for predicting H. pylori infection is also useful in Korea, and the two most important findings suggesting current infection were nodularity and diffuse redness.
Acknowledgements
This work was supported by the funding from Korean College of Helicobacter and Upper Gastrointestinal Research fund 2020 (KCHUGR-202001004). The funding provided only financial support for data analysis without intervention in any part of the study process.
Notes
No potential conflict of interest relevant to this article was reported.