Salvage Regimens after Failure of Previous Helicobacter pylori Eradication Therapy: A Systematic Review and Meta-analysis
Article information
Abstract
Background/Aims
As antibiotic resistance increases and new first-line therapies emerge, salvage therapies for Helicobacter pylori (H. pylori) eradication failures are becoming more common and complicated. This study aimed to systematically review overall salvage regimens after previous failure of H. pylori eradication
Materials and Methods
A systematic review of randomized clinical trials evaluating salvage therapies after previous H. pylori eradication failure was performed. A meta-analysis was conducted when an adequate number of studies suitable for grouping was found.
Results
Overall, 36 studies with 77 treatment arms were identified, and they were highly heterogeneous regarding previously failed regimens and salvage regimens under comparison. Bismuth quadruple therapy after failure of standard triple therapy showed a pooled intention-to-treat (ITT) eradication rate of 75.5% (95% CI, 71.6~79.1%), and the rates were significantly higher with 14-day therapy than 7-day therapy by 9% (95% CI, 2~15%). Levofloxacin triple therapy after failure of standard triple therapy demonstrated a pooled ITT eradication rate of 73.3% (95% CI, 68.4~77.3%). In direct comparison, the two regimens were not significantly different in eradication rates. No study evaluated salvage regimens after the failure of bismuth or non-bismuth quadruple therapy.
Conclusions
The current studies regarding salvage regimens are highly heterogeneous. Bismuth quadruple therapy and levofloxacin triple therapy may be a reliable option after failure of standard triple therapy, but the regional profile of antibiotic resistance should be considered. Further studies are needed for salvage regimens after failure of non-bismuth or bismuth quadruple therapy.
INTRODUCTION
Helicobacter pylori (H. pylori) infection is decreasing but still highly prevalent in Korea with a seroprevalence of 51% in 2015~2016 [1]. Eradication of H. pylori is associated with prevention of peptic ulcer recurrence and regression of gastric mucosa-associated lymphoid tissue lymphoma [2]. In addition, H. pylori eradication can reduce the risk of metachronous recurrence in patients with early gastric cancer who underwent endoscopic resection and the risk of gastric cancer development in individuals with a family history of gastric cancer in first-degree relatives [3,4].
The Korean guidelines published in 2013 recommended a ‘standard’ triple therapy of proton pump inhibitor (PPI), clarithromycin, and amoxicillin as a first-line regimen [2]. However, with increased resistance to clarithromycin [5], the eradication rate of 7-day standard triple therapy is now only 70% [6]. As a result, prescribing a salvage therapy after one or more failed eradication has become common in everyday practice. Because new regimens such as sequential or concomitant therapy have been suggested as new first-line treatment, choosing rescue therapy became more complicated.
In the Korean guideline, bismuth quadruple therapy was recommended after failure of first-line standard triple therapy [2]. However, this guideline was not based on evidences from systematic reviews. More recent evidence-based guidelines recommend bismuth quadruple therapy or levofloxacin triple therapy as rescue regimens, but the detailed recommendations were slightly different with each other: Toronto guidelines recommended either bismuth quadruple therapy or levofloxacin triple therapy, American College of Gastroenterology guidelines favored bismuth quadruple therapy, and Maastricht V consensus report recommended either bismuth quadruple therapy or fluoroquinolone triple or quadruple therapy [7-9].
Several systematic reviews evaluated salvage regimens for H. pylori infection with specific interest to levofloxacin-based therapy as a rescue regimen [10], comparison of levofloxacin triple therapy versus bismuth quadruple therapy [11], levofloxacin triple therapy as a third line regimen [12], refabutin-based rescue therapy [13], rescue regimens after failure of clarithromycin triple therapy [14], or overall second-line therapy [15]. However, data are still insufficient regarding overall salvage regimens for persistent H. pylori infection.
Therefore, this study aimed to systematically review overall randomized controlled trials (RCTs) evaluating salvage regimens after previous failure of H. pylori eradication therapy and to conduct a meta-analysis evaluating successful eradication rate of each salvage regimen.
MATERIALS AND METHODS
1. Literature search and selection
A systematic bibliographic search was conducted in Ovid-MEDLINE, EMBASE, Cochrane Library, KoreaMed, and KMBASE. Search strategies were constructed using combinations of keywords as follows: (Helicobacter pylori OR Helicobacter infections OR H. pylori infection) AND (failure OR retreatment OR second OR salvage OR nonresponse) AND (eradication). References cited in the relevant articles and reviews were also checked for potential eligibility. Two authors (H.J.Y. and W.G.S.) independently reviewed the search outcomes and selected studies, and disagreements were resolved by discussion between the two authors. Inclusion criteria were 1) a RCT as the study design; 2) adult participants with previous failure of H. pylori eradication treatment as the study population; 3) two or more salvage treatment regimens containing at least one antibiotics plus PPI with different antibiotics, duration, or PPIs as intervention and comparator; 4) a successful eradiation rate as the outcome; 5) published between 2008 and 2018; and 6) written in English or Korean. Exclusion criteria were 1) nonrandomized or observational studies; 2) studies that included children or adolescents; 3) studies comparing agents such as probiotics or adjuvants other than antibiotics or PPI; 4) studies with no eradiation rates reported; 5) duplicated publication; 6) unable to obtain full text; and 7) expert opinion, review, and guidelines. This review was conducted in accordance with Preferred Reporting Items for Systemic Reviews and Meta-Analysis (PRISMA) statements [16].
2. Data extraction
Data were extracted using a pre-specified data extraction table by two independent authors. Variables extracted were as follows: year of publication, country, number of patients overall and in each arm, mean age of patients, gender, previous treatment regimens including treatment duration and specific types and doses of antibiotics and PPI, duration of treatment, PPI type, dose, and schedule, antibiotics dose and schedule, timing of test to confirm eradication, successful eradiate rates by intention-to-treat (ITT) and per-protocol (PP) analyses.
3. Risk of bias in individual studies
The risk of bias in included studies were evaluated using the risk of bias (RoB) tool developed by Cochrane collaboration [17]. The criteria was consisted of randomization, allocation concealment, blinding of participants and personnel, blinding of outcome assessment, incomplete outcome data, and selective outcome reporting, and other bias. Each criterion was recorded as low risk, high risk, or unclear. Two authors independently evaluated, and any discordance was resolved by consensus.
4. Statistical analysis
A meta-analysis was conducted when there were at least three studies reporting eradication rates of a treatment regimen or comparisons suitable for grouping. The pooled eradication rates and 95% CIs of a single salvage regimen were estimated both for ITT and PP analyses. Subgroup analyses were conducted to evaluate whether the eradication rate was different according to the treatment duration of a salvage regimen. The risk difference (RD) and 95% CI were calculated to compare eradication regimens using Review Manager (RevMan, version 5.3.5; Cochrane Collaboration, Copenhagen, Denmark). The heterogeneity of the studies was assessed using I2 statistics. The publication bias was planned but not evaluated because there was no comparison with 10 or more studies.
RESULTS
1. Study identification and selection
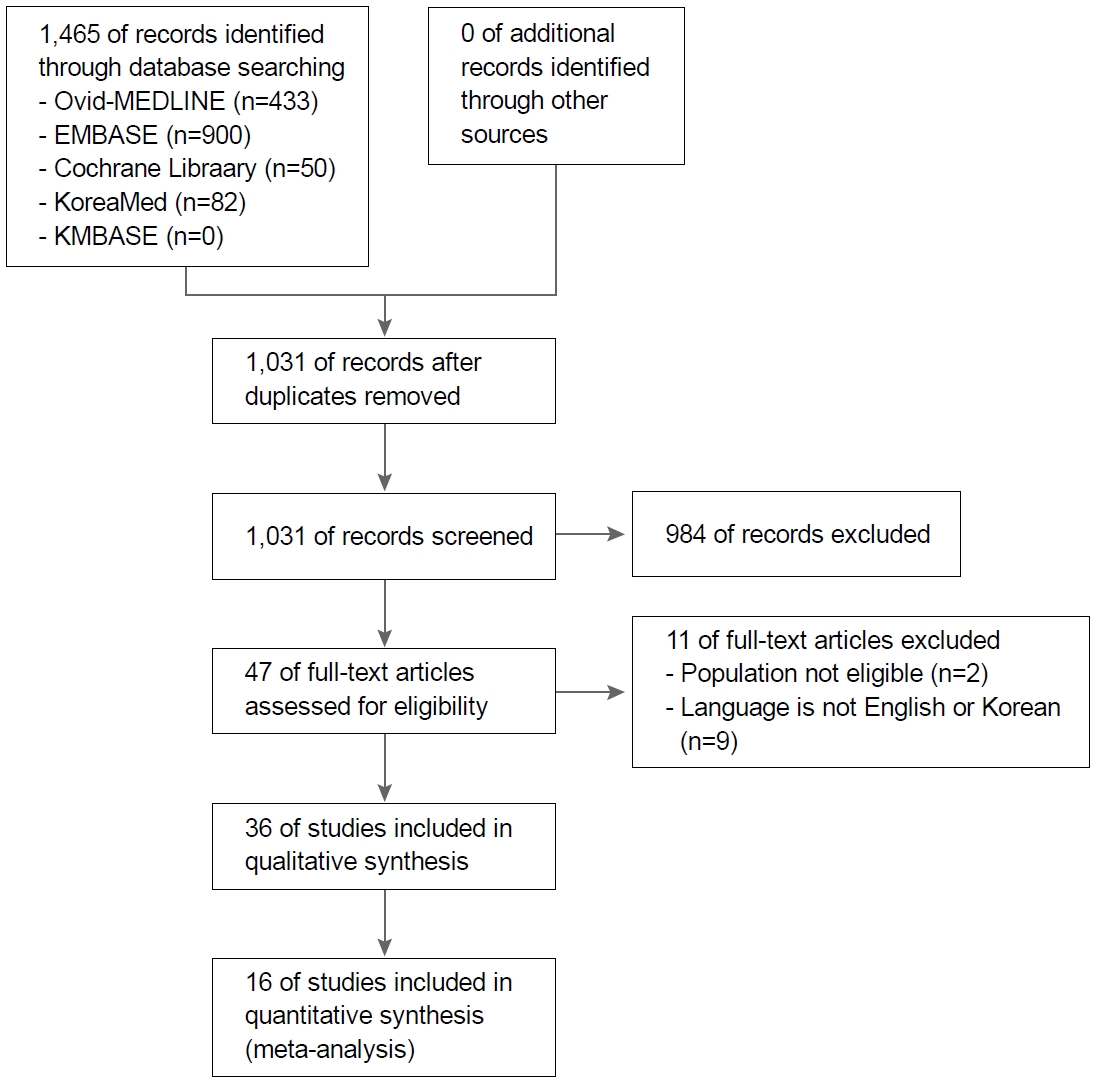
A flow diagram of how relevant studies were selected was presented in Fig. 1. The original database search retrieved 1,465 articles. Among them, 434 articles were duplicated, and 984 studies were excluded by the initial screening by reviewing title and abstract, leaving 47 papers for the full text review. The articles were further excluded because study population were not eligible (n=2) or language was not English or Korean (n=9). As a results, we selected 36 RCTs (with 77 treatment arms) that compared two or more regimens, treatment durations, or PPIs in salvage therapy after at least one failure of previous H. pylori eradication for systematic review [18-53].
2. Characteristics of studies
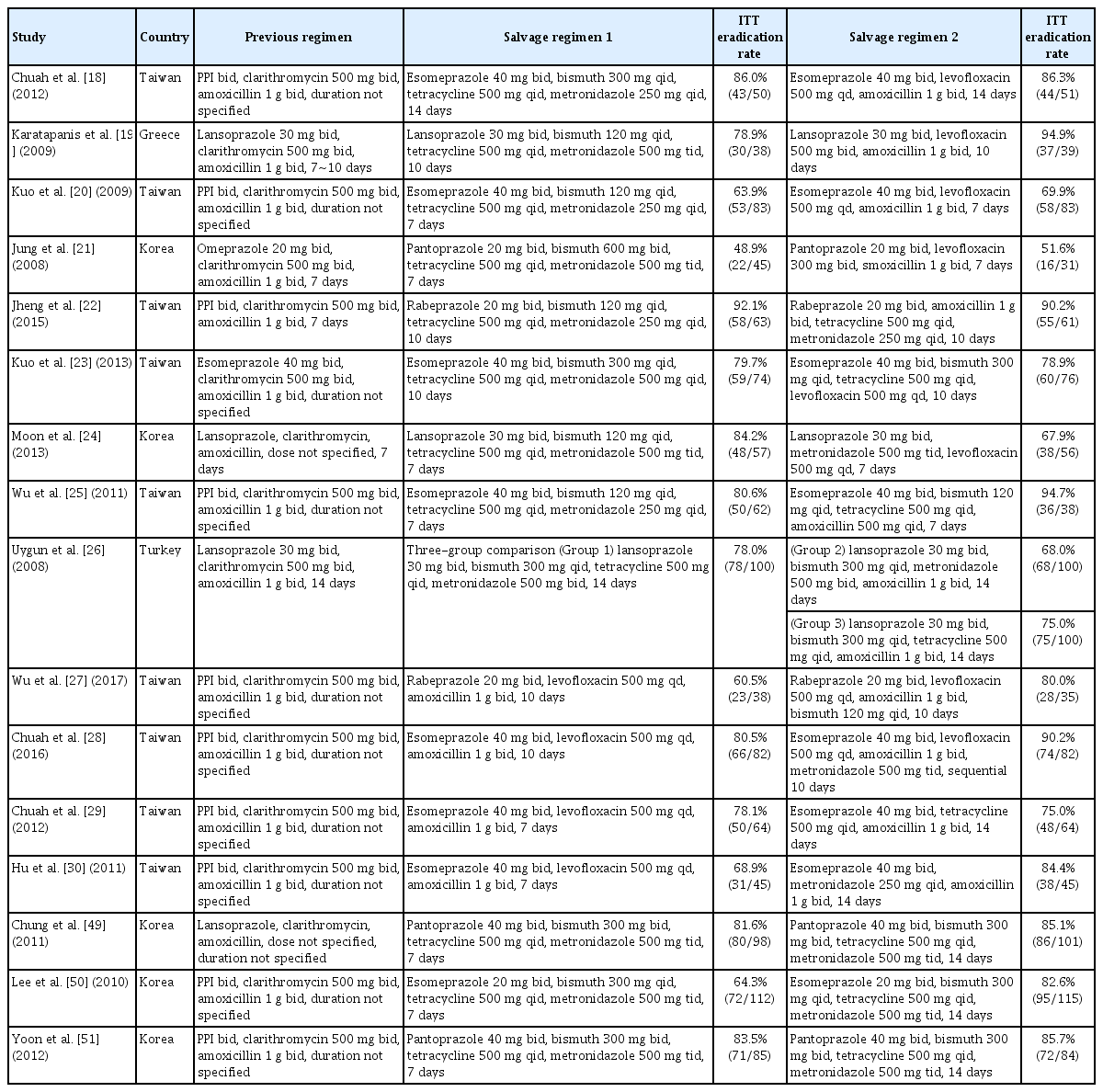
Among the 36 RCTs, 24 studies compared second-line regimens [18-41], five studies compared third-line regimens [42-46], five studies compared different durations of a salvage regimen [47-51], and two studies compared PPIs in a salvage regimen (Supplementary Table 1) [52,53]. These studies were highly heterogeneous with respect to previous failed regimens and salvage regimens under comparison. Of the 24 second-line studies [18-41], 15 studies included patients who failed standard triple therapy [18-32]. There was no study that compared salvage regimens after failure of first-line non-bismuth quadruple therapy (sequential therapy or concomitant therapy), or bismuth quadruple therapy. After failure of standard triple therapy, bismuth quadruple therapy (PPI, bismuth, tetracycline, and metronidazole) was evaluated in nine trials [18-26], and levofloxacin triple therapy (PPI, levofloxacin, and amoxicillin) was in eight trials [18-21,27-30]: four of them directly compared the two regimens [18-21]. The other regimens were evaluated only in one or two studies. No study for third-line treatment evaluated salvage regimens after failure of second-line bismuth quadruple therapy following first-line standard triple or non-bismuth quadruple therapy. Each third-line salvage regimen was evaluated only in one or two studies. Among the five studies compared different treatment durations of a salvage therapy [47-51], three studies compared 7-day versus 14-day bismuth quadruple therapy after failure of first-line standard triple therapy [49-51].
Consequently, a total of 16 RCTs were included in the following four meta-analyses (Table 1): 1) pooled eradication rate of bismuth quadruple therapy after failure of first-line standard triple therapy (n=9) [18-26]; 2) 7-day versus 14-day bismuth quadruple therapy after failure of first-line standard triple therapy (n=3) [49-51]; 3) pooled eradication rate of levofloxacin triple therapy after failure of first-line standard triple therapy (n=8) [18-21,27-30]; and 4) bismuth quadruple therapy versus levofloxacin triple therapy after failure of first-line standard triple therapy (n=4) [18-21].
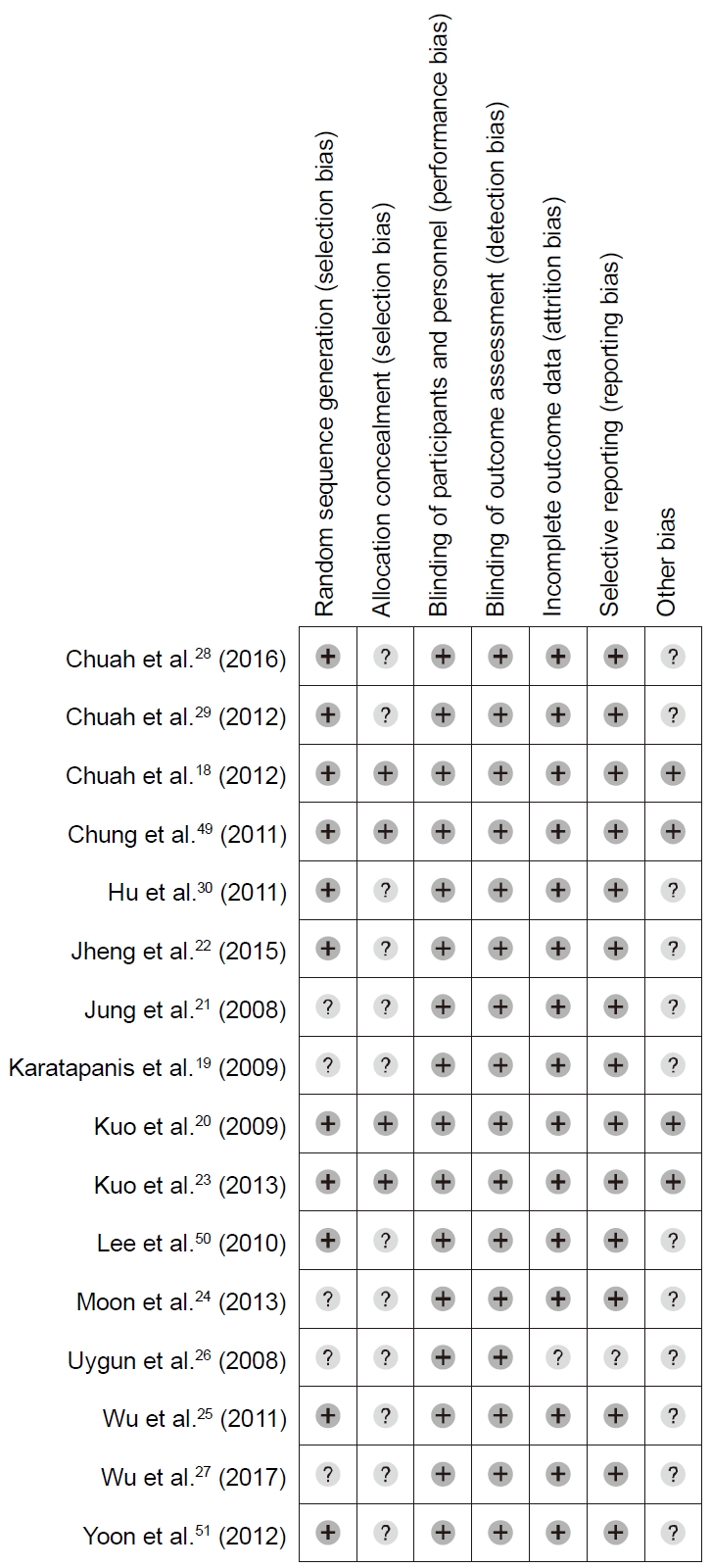
3. Risk of bias in individual studies
Fig. 2 shows the quality assessment of the studies included in the meta-analysis. There was no high risk of bias in the 16 studies. Blinding of participants, investigators, and outcome assessors were adequate in all studies. Incomplete outcome data and selective reporting were unclear only in one study. However, allocation concealment was adequate only in four studies, and random sequence in 11 studies.
4. Bismuth quadruple therapy after failure of standard triple therapy
A total of 572 patients received bismuth quadruple therapy after failure of first-line standard triple therapy in nine studies, and the eradication was successful in 441 patients [18-26]. The pooled eradication rates were 75.5% (95% CI, 71.6~79.1%; I2=78.25%) in ITT analysis and 84.4% (95% CI, 80.7~87.4%; I2=61.8%) in PP analysis (Fig. 3). In the subgroup analyses, the ITT pooled eradication rates were 68.4% (95% CI, 53.0~73.4%; I2=34.5%) after 7-day therapy and 81.6% (95% CI, 76.9~85.6%; I2=83.9%) after 10-14-day therapy (P<0.001). In the PP analysis, successful eradication rates were 80.6% (95% CI, 74.2~85.7%; I2=29.6%) after 7-day therapy and 87.3% (95% CI, 82.9~90.7%; I2=73.8%) after 10-14-day therapy (P<0.001).
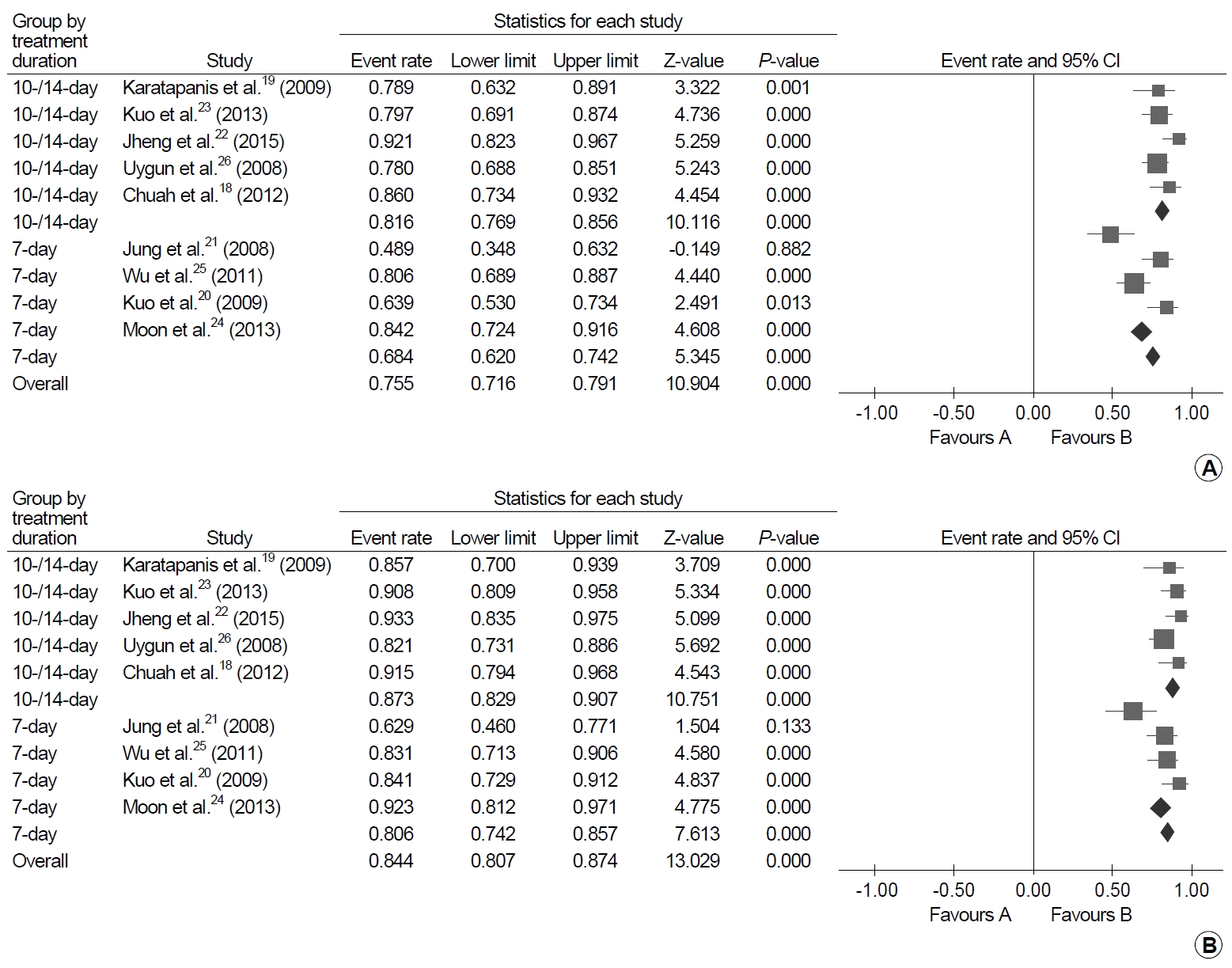
Forest plot of successful eradication rates of bismuth quadruple therapy after failure of standard triple therapy. (A) Intention-to-treat analysis and (B) per-protocol analysis.
In the three RCTs that compared 7-day versus 14-day bismuth quadruple therapy after failure of first-line standard triple therapy, 595 patients were included: of whom 295 were treated with 7-day therapy and 300 were treated with 14-day therapy [49-51]. In the meta-analysis, the eradication rates were significantly higher with 14-day therapy than 7-day therapy by 9% point (95% CI, 2~15%; I2=61%) in ITT analysis and 12% point (95% CI, 1~23%; I2=24%) in PP analysis (Fig. 4).
5. Levofloxacin triple therapy after failure of standard triple therapy
Levofloxacin triple therapy after failure of first-line standard triple therapy was administered to 433 patients in eight studies and was successful in 325 patients [18-21,27-30]. The ITT and PP pooled eradication rates were 73.3% (95% CI, 68.4~77.3%; I2=71.9%) and 76.4% (95% CI, 71.7~80.6%; I2=70.5%), respectively (Fig. 5). The subgroup analyses showed that the eradication rate of 10-14-day therapy was significantly higher than 7-day therapy in the ITT analysis (78.5%; 95% CI, 71.9~84.0%; I2=78.3% vs. 69.1%; 95% CI, 62.6~74.9%; I2=55.0%; P=0.036) but not in the PP analysis (81.3%; 95% CI, 74.3~86.7%; I2=77.0% vs. 73.2%; 95% CI, 66.6~78.9%; I2=60.1%; P=0.076).
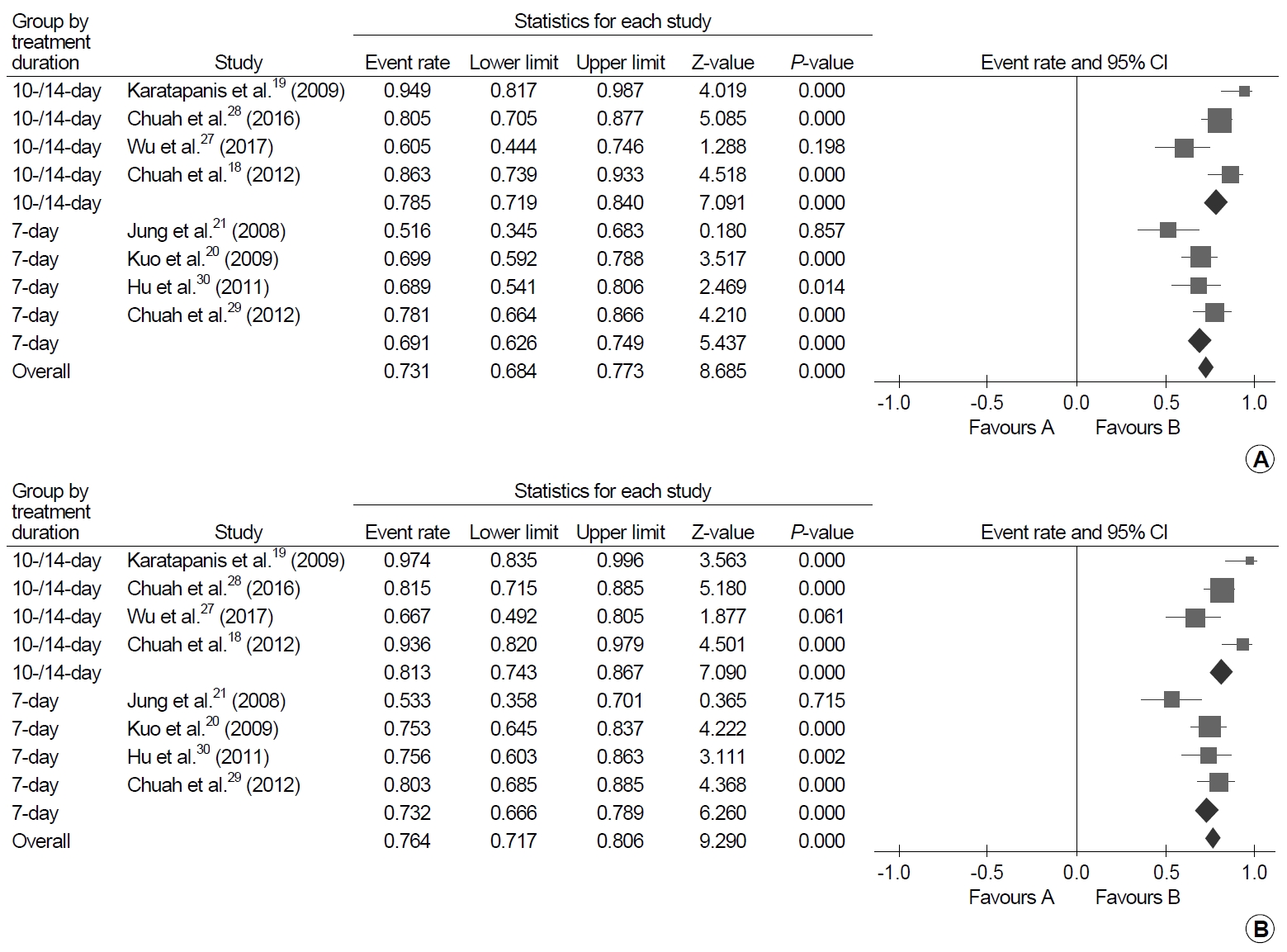
Forest plot of successful eradication rates of levofloxacin triple therapy after failure of standard triple therapy. (A) Intention-to-treat analysis and (B) per-protocol analysis.
In a factorial RCT that was included in the systematic review, the authors compared 7-day versus 10-day therapy and levofloxacin 500 mg once versus twice daily in the levofloxacin triple therapy as a rescue after failure of first-line PPI, clarithromycin, and either amoxicillin or metronidazole [48]. In the results, 10-day therapy showed a higher ITT eradication rate of 87.5% (70/80) than 7-day therapy of 67.5% (54/80) (P<0.005), while there was no difference in the eradiation rates between levofloxacin 500 mg once versus twice daily.
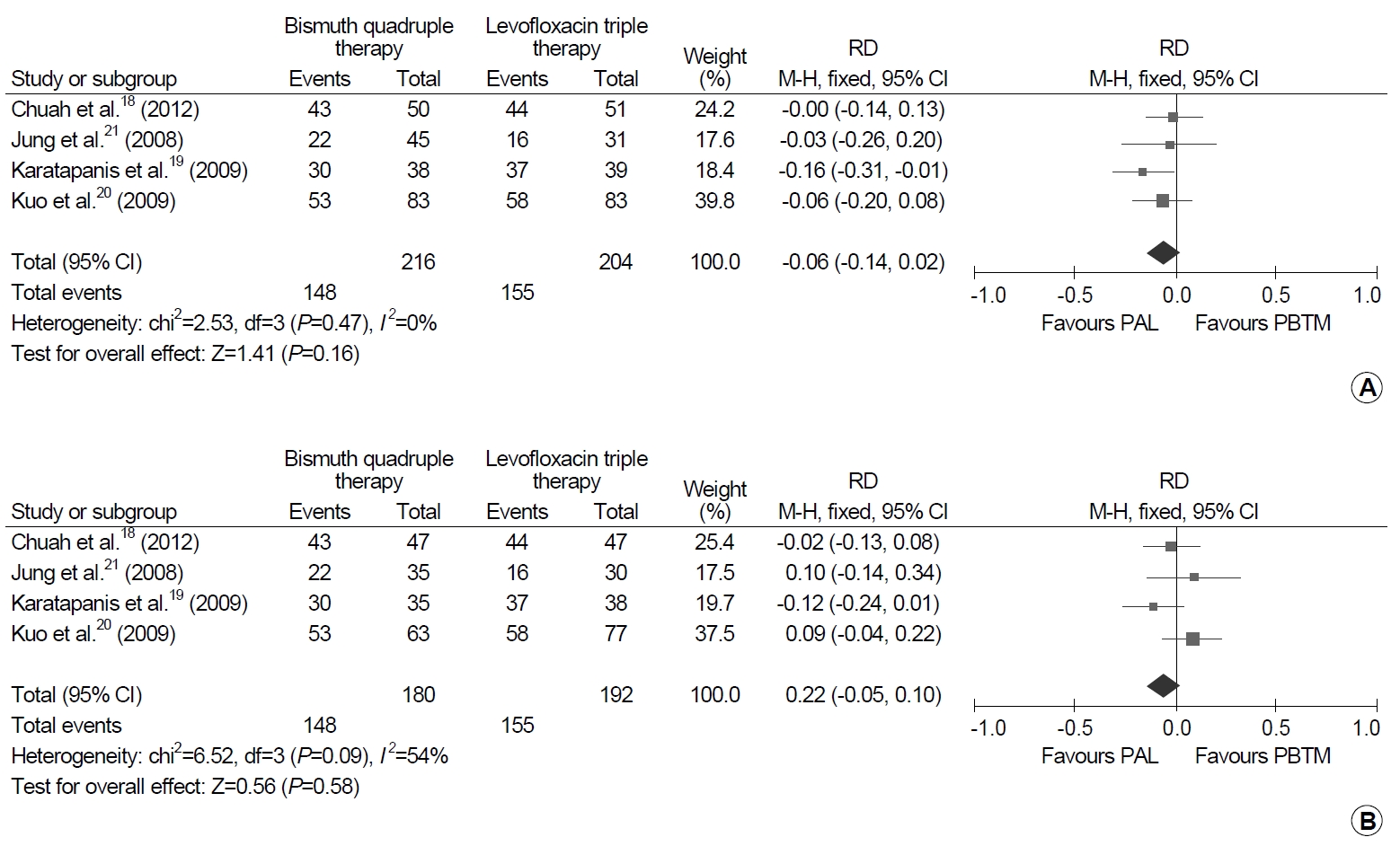
6. Bismuth quadruple therapy vs. levofloxacin triple therapy after failure of standard triple therapy
Bismuth quadruple therapy and levofloxacin triple therapy were directly compared as second-line regimens after failure of first-line standard triple therapy in 420 patients in four trials: 216 patients received bismuth quadruple therapy and 204 patients received levofloxacin triple therapy (Fig. 6) [18-21]. There was no significant difference in the eradication rates both in ITT and PP analyses. In detail, there was a tendency of favoring levofloxacin triple therapy over bismuth quadruple therapy by 6% point (95% CI, -2% to 14%; I2=0%; P=0.160) in the ITT analysis, but a tendency of favoring bismuth quadruple therapy over levofloxacin triple therapy by 2% point (95% CI, -5% to 10%; I2=54%; P=0.580).
DISCUSSION
In this study, we systematically reviewed salvage therapies after failure of previous H. pylori eradication treatment and found that the regimens were highly heterogeneous. Among them, evidences were relatively sufficient for 14-day bismuth quadruple therapy and 10-day levofloxacin triple therapy after failure of first-line standard triple therapy. On the contrary, there was no study that evaluated salvage regimens after failure of bismuth or non-bismuth quadruple therapy.
In our systematic review, studies were sufficiently robust to support second-line salvage regimen after failure of standard triple therapy. Nine and eight RCTs evaluated bismuth quadruple therapy and levofloxacin triple therapy, respectively, and four of them directly compared the two treatments. The pooled eradication rates of the regimens were comparable to each other, and particularly, there was no significant difference in the successful eradication rates between the two regimens. In previous meta-analyses, 10-day levofloxacin triple therapy demonstrated higher eradiation rate than 7-day bismuth quadruple therapy [10,11]. It seems that the two regimens showed similar efficacy because they were administered for the same duration at each study in our analysis (7, 10, or 14 days each). However, before applying these results to our practice, it is necessary to consider the pattern of H. pylori antibiotic resistance in Korea. In a recent nationwide study, the prevalence of levofloxacin-resistant H. pylori strain was 37.0% [5]. The efficacy of levofloxacin triple therapy is substantially compromised for H. pylori with levofloxacin resistance [18]. Therefore, in Korea, bismuth quadruple therapy, rather than levofloxacin triple therapy, can be recommended as a second-line treatment after failure of standard triple therapy. This is consistent with the previous Korean guidelines [2]. However, this guideline lacked the recommendation on the duration of this regimen. In our analysis, 10-/14-day treatment showed >80% ITT and >85% PP eradication rates in the subgroup analysis, and 14-day treatment showed significantly higher eradication rates than 7-day treatment in the direct comparison. In the abovementioned study, the prevalence of metronidazole resistance was also high as 29.5% in Korea [5]. Increasing duration and dose of metronidazole can overcome the metronidazole resistance [54]. Therefore, 14-day bismuth quadruple therapy would be most suitable option for the salvage treatment after failure of standard triple therapy in Korea.
The evidence regarding salvage regimen after failure of non-bismuth or bismuth quadruple therapy was very weak in our study because no RCT was identified in the literature review. Similarly, recent guidelines provided recommendations based on the meta-analyses of cohort studies [7-9]. Levofloxacin triple therapy showed 81% (95% CI, 71~91%) eradication rate after failure of non-bismuth quadruple therapy in a meta-analysis of 86 patients in five observational studies [14]. Meanwhile, bismuth quadruple therapy presented 85% (95% CI, 63~100%) eradication rate after failure of non-bismuth quadruple therapy in another meta-analysis of two studies of unknown number of patients [7]. Thus, bismuth quadruple therapy may be recommended as a salvage regimen after failure of non-bismuth quadruple therapy in Korea because of high levofloxacin resistance rate. Nevertheless, levofloxacin triple therapy may be considered after failure of bismuth quadruple therapy. A meta-analysis of 501 patients in five cohort studies conducted in Maastricht V report showed 70.0% (95% CI, 62.4~76.6%) eradication rate of third-line levofloxacin triple therapy [7]. In a recent Korean retrospective cohort study, the eradication rate of third-line levofloxacin triple therapy was 56.9% (62/109) [55]. Taken together with these studies and guidelines, our study emphasizes the urgent need for the studies regarding salvage regimens after failure of non-bismuth or bismuth quadruple therapy. Potential new salvage regimens may include levofloxacin-bismuth therapy (PPI, amoxicillin, levofloxacin, bismuth) that is expected to overcome levofloxacin resistance using bismuth [27,36].
There are several limitation in our study. First, we included RCTs only, but this strategy may not appropriate to evaluate salvage regimens after failure of non-bismuth quadruple therapy because this regimen was introduced more relatively than the standard triple therapy. Second, the studies included in the review were highly heterogeneous and the number of studies included in the meta-analysis was small that publication bias was not assessed. Third, only five of 16 studies that were included in the meta-analysis were conducted in Korea, which compromised applicability of study results to Korean patients. The efficacy of H. pylori eradication can be affected by regional differences in antibiotic resistance. So, although levofloxacin triple therapy showed similar efficacy to bismuth quadruple therapy in the meta-analysis, this regimen may not be recommended as a salvage regimen after failure of standard triple therapy in Korea because of high H. pylori resistance rate to levofloxacin.
In conclusion, the present systematic review showed that the current studies regarding salvage regimens were highly heterogeneous. Bismuth quadruple therapy and levofloxacin triple therapy may be reliable option after failure of standard triple therapy, but regional profile in antibiotics resistance should be considered. Further studies are needed to evaluate salvage regimens after failure of non-bismuth or bismuth quadruple therapy.
Acknowledgements
We would like to express our deep gratitude to Mi-young Choi, PhD of National Evidence based Healthcare Collaborating Agency who performed initial literature search for systematic review, Ein Soon Shin, PhD & MPH, Research Head of the Research Agency for Clinical Practice Guidelines of Korean Academy of Medical Sciences for the Meta-Analysis Workshop.
Notes
No potential conflict of interest relevant to this article was reported.
Supplementary Materials
Characteristics of Overall Salvage Regimens Identified in the Systematic Review