|
 |
- Search
| Korean J Helicobacter Up Gastrointest Res > Volume 21(2); 2021 > Article |
|

See editorial "Considerations for Endoscopic Treatment of Undifferentiated-type Early Gastric Cancer" in Volume 21 on page 103.
Abstract
Background/Aims
The prediction of invasion depth is important to decide the treatment modality for undifferentiated-type early gastric cancer (EGC) less than 20 mm in size without ulceration. We aimed to identify the endoscopic features associated with submucosal invasion in undifferentiated-type EGC that meet the criteria of size and ulcer status.
Methods
A total of 120 patients with undifferentiated-type EGC who underwent endoscopic submucosal dissection (ESD) or gastrectomy between August 2008 and December 2017 were enrolled and reviewed retrospectively. All lesions met the ESD indications except for the invasion depth. We analyzed the endoscopic features of the tumors before resection and invasion depth after resection.
Results
There were 97 mucosal and 23 submucosal cancer lesions. Multivariable analysis revealed that the polypoid (OR, 90.8; 95% CI, 3.5~2,346.2) or elevated (OR, 5.0; 95% CI, 1.2~21.3) types, deep depression (OR, 76.0; 95% CI, 4.5~1,284.6), and upper (OR, 22.7; 95% CI, 3.0~170.8) or middle location (OR, 10.3; 95% CI, 1.9~55.4) were significant risk factors of submucosal invasion.
Endoscopic resection has been widely used in the treatment of early gastric cancer (EGC) with negligible risk of lymph node (LN) metastasis [1]. Endoscopic submucosal dissection (ESD) provides benefits such as minimal invasion, low cost, and keeping the quality of life by the preservation of the stomach compared to the surgical resection. However, the oncologic outcome of the two treatment modalities is similar [2]. Therefore, ESD is considered a more suitable treatment option for EGC when the risk of LN metastasis is extremely low and lesion size and location seem to be appropriate to achieve en bloc resection.
The indication for ESD is limited to the differentiated-type mucosal cancer (T1a), sized less than 20 mm with no ulceration. This is the absolute indication in Japanese and Korean gastric cancer treatment guidelines [3,4]. Several studies have demonstrated that it is acceptable to expand the ESD indication to lesions clinically diagnosed as mucosal cancer (T1a) and 1) differentiated-type, no ulcer, regardless of tumor size; 2) differentiated-type, presence of ulcer, size less than 30 mm; and 3) undifferentiatedtype, no ulcer, size less than 20 mm (expanded indication) [5-7]. Thus, accurate prediction of the invasion depth of EGC is crucial to determine the treatment modality when the lesion size and ulcer status meet the ESD indication. Morphology of EGC used to evaluate the invasion depth showed approximately 72% to 78% accuracy of depth prediction [8,9]. Additional endoscopic ultrasonography (EUS) after endoscopy can also be helpful in deciding the optimal treatment for differentiated-type EGC with submucosal invasive features [10,11]. Unfortunately, the accuracy of EUS for undifferentiated-type EGC has been reported to be worse than that for differentiated-type EGC [9]. The previous study to predict the invastion depth in undifferentiated-type EGC included lesions regardless of size and ulcer status. Hence, the use of endoscopic features to predict the invasion depth in undifferentiated-type EGC is important, especially when the other factors except invasion depth meet ESD indication.
Therefore, we aimed to identify the endoscopic features associated with submucosal invasion for undifferentiated-type EGC sized less than 20 mm without ulceration.
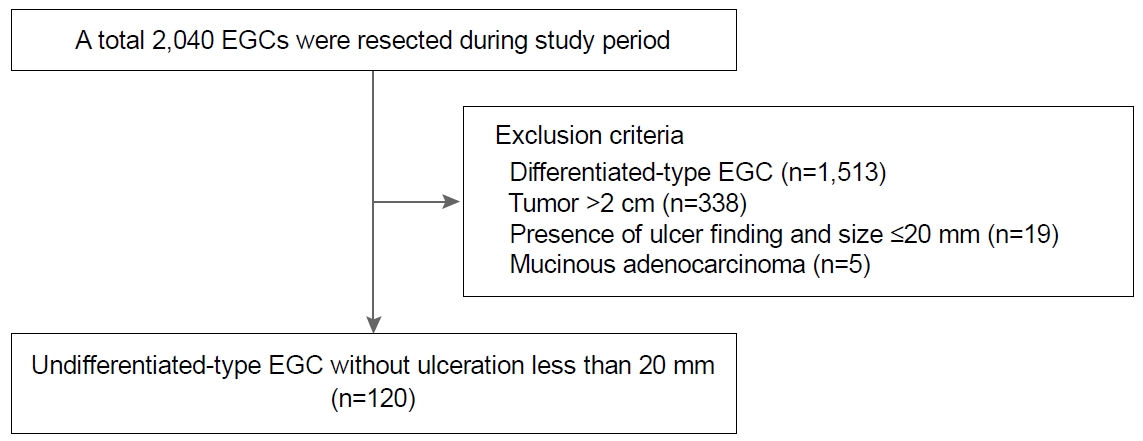
Between January 2010 and December 2017, patients who underwent laparoscopic gastrectomy or ESD for EGC at Pusan National University Yangsan Hospital in Korea, were considered for inclusion in this study. Exclusion criteria were: differentiated-type histology (n=1,513), lesion diameter >20 mm (n=338), presence of an active ulcer or scar (n=19) and mucinous adenocarcinoma (n=5). Finally, 120 undifferentiated-type EGC lesions in 120 patients that met the size and ulcer status of the expanded ESD indication were included and reviewed retrospectively (Fig. 1). There was no evidence of LN enlargement in the abdominal computed tomography for all included patients. All lesions had been treated by ESD and/or laparoscopic gastrectomy with D1+ LN dissection. Written informed consent was obtained from all patients before the treatment. This study was approved by the Ethics Committee of the Institutional Review Board of the Pusan National University Yangsan Hospital (IRB No. 05-2018-165).
We reviewed the recorded images of the esophagogastroduodenoscopy. The endoscopic features of the lesions are based on the images before the biopsy, because the endoscopic biopsy can induce the change of tumor morphology. When it was difficult to identify the endoscopic features of EGC, we used the images obtained by using the GIF-H260 or GIF-HQ290 (Olympus, Tokyo, Japan) in Pusan National University Yangsan Hospital. The tumor gross type was classified according to the Paris classification of superficial neoplastic lesions as type 0-I (polypoid), 0-IIa (superficial elevated), 0-IIb (flat), and 0-IIc (superficial depressed) [12]. We categorized the lesions into four endoscopic findings. ‘Subtule protrusion/depression’ was a smooth elevation/depression without deep depression or nodularity (Fig. 2A). ‘Nodular change’ was a raised nodular surface (Fig. 2B). ‘Fold change’ was fold abrupt cutting or clubbing converging fold (Fig. 2C). ‘Deep depression’ was defined as the opposite to the subtle depression (Fig. 2D) [8]. The color of the tumor surface was categorized into discoloration, redness, and other changes. ‘Discoloration’ was defined as a pale color compared to the mucosa surrounding the lesion. ‘Redness’ was the reddish tint compared to the surrounding mucosa color. ‘Other changes’ includes the whitish or yellowish color, and no changes. All lesions were analyzed by the consensus of two endoscopists (Kim SJ and Choi CW) without any pathologic information. Two endoscopists had experiences of more than 300 endoscopic resection cases.
The location of the tumor was defined by the Japanese classification of gastric cancer as upper, middle, and lower third [13]. Undifferentiated-type EGC includes signet ring cell carcinoma and poorly-differentiated carcinoma according to the Japanese gastric cancer treatment guidelines [3], although mucinous carcinoma is also classified as undifferentiated type. Undifferentiated-type dominant mixed histology is classified as undifferentiated type. Histologic evaluation was performed after hematoxylin-eosin staining for serial sections of specimens. Slice thickness was 2 mm for ESD specimens and 4 mm for surgical specimens. The largest diameter was used to evaluate tumor size.
The endoscopic resections were performed using three kinds of endoscopes (GIF Q260J, GIF HQ290, GIF 2TQ260M; Olympus) with a transparent cap attached at the distal end. The steps in the ESD technique were: first, markings were made around the lesions, 5 mm apart from the suspected tumor margin. Second, the submucosal injection was administered using a saline solution mixed with glycerin, epinephrine, and indigo carmine. Subsequently, a circumferential incision was made surrounding the lesion with an ESD knife. Lastly, the submucosal layer beneath the lesion was dissected.
Laparoscopic gastrectomy with D1+ LN dissection was performed. D1+ LN dissection includes the perigastric area (No. 1-7), common hepatic artery (No. 8a), and celiac axis (No. 9). For proximal gastric cancer, an additional LN dissection was performed along the proximal splenic artery (No. 11p) [3].
The chi-square or Fisher’s exact test was used to assess the associations among categorical variables. The student’s t test or Mann-Whitney test was used for continuous variables. Statistical significance was set at P<0.05. Variables with a value of P<0.05 on univariate analysis were included in a backward likelihood multiple logistic regression model to identify the endoscopic features that were associated with submucosal invasion of undifferentiated-type EGC. All analyses were performed with SPSS version 21.0 (SPSS, Chicago, IL, USA).
Patients consisted of 58 men and 62 women with a mean age of 59.8±11.4 years. The mean tumor size was 13.7 mm. Among 120 lesions with undifferentiated type EGC, 46 lesions were pure signet ring cell carcinomas, 39 were pure poorly differentiated adenocarcinomas (Table 1). During the study period, 14 patients received ESD as the treatment for undifferentiated-type EGC. The additional gastrectomy with LN dissection after ESD was performed for six patients. In total, 112 patients received the laparoscopic gastrectomy with LN dissection. LN metastasis was found in the four patients who received the surgical resection (three mucosal cancer and one submucosal cancer). All patients with LN metastasis were beyond ESD curative resection criteria because of the presence of lymphovascular invasion on the resected specimens.
Among the 120 undifferentiated-type EGC patients, the invasion depth was mucosa in 97 lesions (80.8%) and submucosa in 23 lesions (19.2%). The mean age of patients was significantly lower for the mucosal cancer compared to the submucosal cancer (58.3±11.4 years vs. 66.2±9.1 years, P=0.003). In the univariable analysis, the proportion of polypoid, elevated, flat and depressed gross types in the mucosal cancer was significantly different compared to that in submucosal cancer (P<0.001). In endoscopic findings, the presence of deep depression (1.0% vs. 21.7%, P=0.001) was significantly prevalent in the submucosal cancer. Upper, middle and lower locations were significantly different between mucosal and submucosal cancers (P<0.001) (Table 2).
Multivariate analysis showed that the polypoid (OR, 90.8; 95% CI, 3.5~2,346.2), elevated (OR, 5.0; 95% CI, 1.2~21.3) gross type, the presence of deep depression (OR, 76.0; 95% CI, 4.5~1,284.6), and upper (OR, 22.7; 95% CI, 3.0~170.8) and middle (OR, 10.3; 95% CI, 1.9~55.4) third location of the stomach were associated with the submucosal invasion (Table 3).
ESD has been used widely to treat EGC with negligible risk of LN metastasis. Endoscopic resection is absolutely indicated for differentiated-type EGC sized less than 20 mm without ulceration [1]. A recent prospective study demonstrated that ESD could be accepted as a curative treatment for patients with EGC who meet the expanded criteria [14]. The accurate prediction of invasion depth of EGC is mandatory in order to select the appropriate treatment for patients. However, the accuracy of endoscopic prediction for invasion depth ranged approximately from 72% to 85%. The diagnostic yield of advanced techniques including EUS and magnifying endoscopy with narrow-band imaging are also unsatisfactory. Reported accuracy ranged from 79% to 84% by EUS and 81% by magnifying endoscopy [8,10,11,15]. In undifferentiated-type EGC, the prediction of tumor depth is especially difficult compared to differentiated-type EGC. Therefore, we tried to identify the predictive factors of submucosal invasion in undifferentiated-type EGC that met ESD indication regardless of invasion depth. In the present study, the polypoid/elevated gross type, presence of deep depression, and middle to upper location were associated with submucosal invasion. Several studies reported that submucosal invasion increased the risk of LN metastasis in undifferentiated-type EGC [16,17]. In 112 patients who received the surgical resection, LN metastasis was found 3.4% (3/89) in mucosal cancer and 4.3% (1/23) submucosal cancer. All patients with LN metastasis had lymphovascular invasion that is a more potent risk factor of LN metastasis [16,17].
In patients with polypoid or elevated gross-type EGC, the prevalence of submucosal cancer was higher (39.6%, 21/53) than that of other gross types (1.9% [1/53] in flat type, 7.4% [1/14] in depressed type). Flat or superficial spreading lesions are regarded as an endoscopic feature suggestive of mucosal cancer [8]. Depressed-type EGCs usually include lesions with active ulceration or severe fibrosis, which are endoscopic features associated with submucosal invasion. We excluded lesions with active ulceration or fibrosis that are beyond of expanded ESD indication. The result of present study is consistent with the previous study, in terms of gross type, which reported that an elevated type of EGC was associated with deep submucosal invasion [11]. Especially, the polypoid gross type was the most important predictive factor of submucosal invasion in this study. The published data from different investigators demonstrated that subepithelial tumor-like protrusion can be regarded as a submucosal cancer [8,10]. Our finding of a higher risk of submucosal invasion in lesions with polypoid morphology is consistent with these results.
According to our endoscopic findings, the presence of deep depression is associated with submucosal invasion. The deep depression means mucosal damage that does not extend deeper through the submucosa. An ulcer can occur as the damage progresses through the muscularis mucosa into the deeper layers. Ulcerative EGCs have a higher risk of submucosal invasion and LN metastasis [18]. However, deep depression is not a characteristic of submucosal cancer in differentiated-type EGC. One possible explanation of this result is a different tumor-spreading pattern of the undifferentiated-type histology compared to the differentiated-type. Signet ring cell carcinoma shows a diffuse infiltration of tumor cells individually or in small nests [19]. Poorly differentiated adenocarcinoma, especially the non-solid type, displays diffuse infiltration and rapid progression [20]. These differences can be the reasons for a higher incidence of submucosal invasion in the undifferentiated-type EGCs with deep depression. In the previous study about early gastric signet ring cell carcinoma, deep depression was also associated with submucosal invasion [21].
This study demonstrated that the middle-to-upper location is associated with submucosal invasion. The mechanism for the relationship between the location and submucosal invasion in this study is uncertain. There can be two possible explanations. First, the wall thickness of the middle-to-upper third is thinner than that of the lower third [22]. Therefore, EGC located in the middle-to-upper third may invade the submucosa layer more rapidly than the lower third. Second, middle-to-upper third of the stomach have several blind spots such as the lesser curvature side of cardia and posterior wall of the body. Inadequate air insufflation may be the cause of a missed lesion located on the body’s greater curvature. Thus, delayed detection could cause the more invasive cancer.
The strength of this study is that we evaluated the endoscopic features of submucosal cancer only in undifferentiated-type EGC sized less than 20 mm without ulceration. One of the valuable findings of this study is that the presence of deep depression in undifferentiated-type EGC had a higher risk of submucosal invasion.
There are several limitations in the present study. First, this is a retrospective, single-center study. The sample size is small to definitely confirm these predictive factors. The lesions with deep depression associated with submucosal invasion was only six cases (5%). Therefore, prospective multicenter studies are needed to overcome this limitation. However, endoscopic features to predict the invasion depth identified in this study may be helpful for future studies and provide some information for clinicians to make treatment decision. Second, only 14 cases of ESD were included in this study. There is a possibility that there has been an overestimation of the specimen size because of specimen stretching during fixation. This potentially limits the accuracy of the tumor size. However, the expanded indication for EGC is based on surgical specimens. Therefore, our findings can be applied to the lesions found during the endoscopic examination. Finally, we demonstrated the results about the information obtained by white light endoscopy. EUS was performed only for 21 patients with 81% (17/21) accuracy. All cases were an under staging of invasion depth. This is consistent with data from a previous study [23]. We did not perform magnifying endoscopy for the purpose of assessing invasion depth.
In conclusion, we found that the polypoid or elevated gross type, presence of deep depression, and middle-to-upper third location were associated with submucosal invasion in undifferentiated-type EGC sized less than 20 mm without ulceration. Thus, the decision of ESD for undifferentiated-type EGC with these features must be made carefully, although the size and ulcer status meet the expanded ESD indication.
Fig. 2.
Classification of endoscopic findings. (A) Subtle protrusion/depression (mucosal cancer). (B) Nodular change (mucosal cancer). (C) Fold change (mucosal cancer). (D) Deep depression (submucosal cancer).
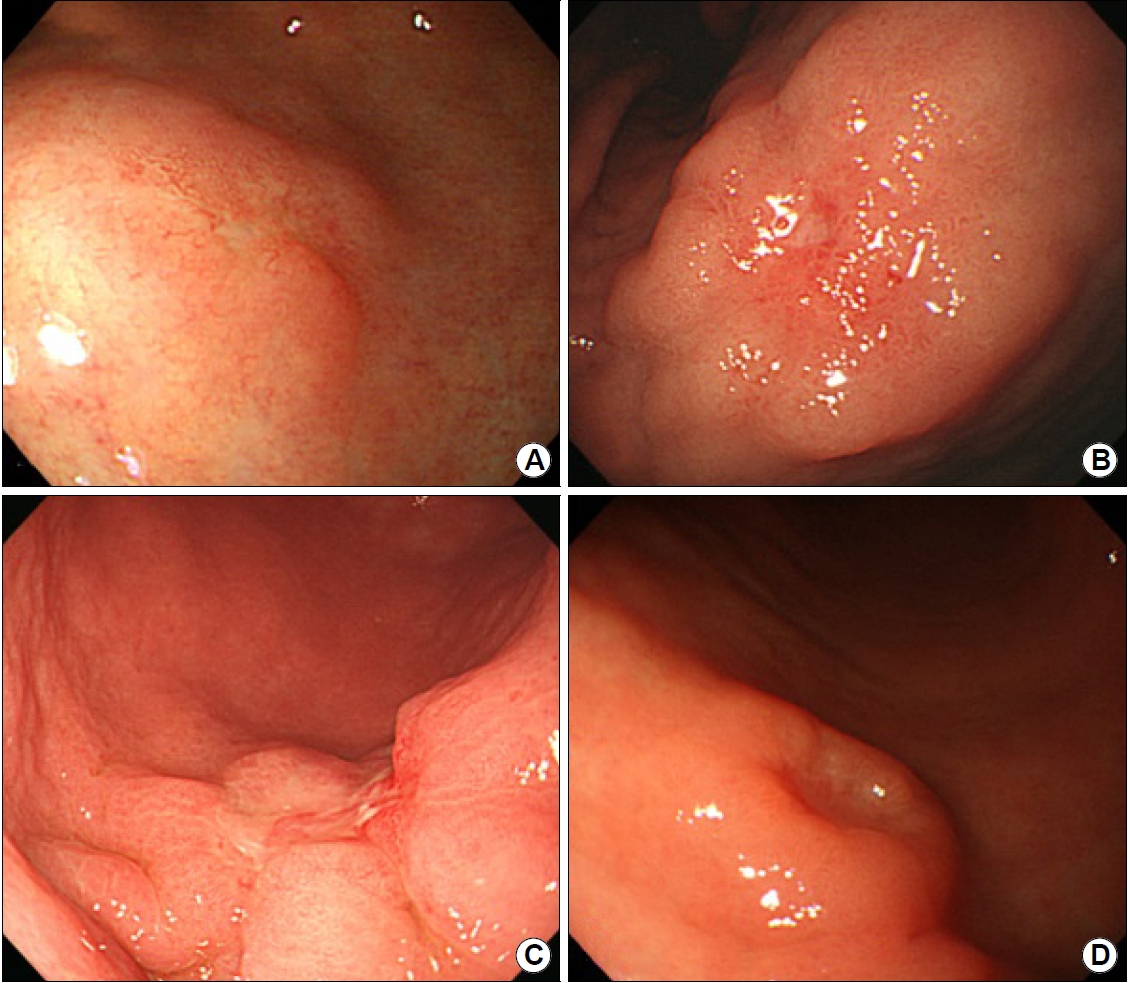
Table 1.
Baseline Characteristics (n=120)
Table 2.
Comparison of Tumor Characteristics between Mucosal and Submucosal Invasive Cancer
Table 3.
Multivariate Analysis to Evaluate the Predictive Factors of Submucosal Invasion
REFERENCES
1. Ono H, Yao K, Fujishiro M, et al. Guidelines for endoscopic submucosal dissection and endoscopic mucosal resection for early gastric cancer. Dig Endosc 2016;28:3-15.


2. Choi JH, Kim ES, Lee YJ, et al. Comparison of quality of life and worry of cancer recurrence between endoscopic and surgical treatment for early gastric cancer. Gastrointest Endosc 2015;82:299-307.


3. Japanese Gastric Cancer Association. Japanese gastric cancer treatment guidelines 2014 (ver. 4). Gastric Cancer 2017;20:1-19.


4. Lee JH, Kim JG, Jung HK, et al. Synopsis on clinical practice guideline of gastric cancer in Korea: an evidence-based approach. Korean J Gastroenterol 2014;63:66-81.


5. Gotoda T, Yanagisawa A, Sasako M, et al. Incidence of lymph node metastasis from early gastric cancer: estimation with a large number of cases at two large centers. Gastric Cancer 2000;3:219-225.



6. Choi MK, Kim GH, Park DY, et al. Long-term outcomes of endoscopic submucosal dissection for early gastric cancer: a single-center experience. Surg Endosc 2013;27:4250-4258.



7. Jeon HK, Lee SJ, Kim GH, Park DY, Lee BE, Song GA. Endoscopic submucosal dissection for undifferentiated-type early gastric cancer: short- and long-term outcomes. Surg Endosc 2018;32:1963-1970.



8. Choi J, Kim SG, Im JP, Kim JS, Jung HC, Song IS. Endoscopic prediction of tumor invasion depth in early gastric cancer. Gastrointest Endosc 2011;73:917-927.


9. Choi J, Kim SG, Im JP, Kim JS, Jung HC, Song IS. Comparison of endoscopic ultrasonography and conventional endoscopy for prediction of depth of tumor invasion in early gastric cancer. Endoscopy 2010;42:705-713.



10. Tsujii Y, Kato M, Inoue T, et al. Integrated diagnostic strategy for the invasion depth of early gastric cancer by conventional endoscopy and EUS. Gastrointest Endosc 2015;82:452-459.


11. Kim SJ, Choi CW, Kang DH, et al. Factors associated with the efficacy of miniprobe endoscopic ultrasonography after conventional endoscopy for the prediction of invasion depth of early gastric cancer. Scand J Gastroenterol 2017;52:864-869.


12. Endoscopic Classification Review Group. Update on the paris classification of superficial neoplastic lesions in the digestive tract. Endoscopy 2005;37:570-578.


13. Japanese Gastric Cancer Association. Japanese classification of gastric carcinoma: 3rd English edition. Gastric Cancer 2011;14:101-112.



14. Kim SG, Park CM, Lee NR, et al. Long-term clinical outcomes of endoscopic submucosal dissection in patients with early gastric cancer: a prospective multicenter cohort study. Gut Liver 2018;12:402-410.




15. Kikuchi D, Iizuka T, Hoteya S, et al. Usefulness of magnifying endoscopy with narrow-band imaging for determining tumor invasion depth in early gastric cancer. Gastroenterol Res Pract 2013;2013:217695.




16. Ye BD, Kim SG, Lee JY, et al. Predictive factors for lymph node metastasis and endoscopic treatment strategies for undifferentiated early gastric cancer. J Gastroenterol Hepatol 2008;23:46-50.


17. Hirasawa T, Gotoda T, Miyata S, et al. Incidence of lymph node metastasis and the feasibility of endoscopic resection for undifferentiated-type early gastric cancer. Gastric Cancer 2009;12:148-152.



18. Lee YJ, Kim JH, Park JJ, et al. The implications of endoscopic ulcer in early gastric cancer: can we predict clinical behaviors from endoscopy? PLoS One 2016;11:e0164339.



19. Kim H, Kim JH, Lee YC, et al. Growth patterns of signet ring cell carcinoma of the stomach for endoscopic resection. Gut Liver 2015;9:720-726.



20. Kunisaki C, Akiyama H, Nomura M, et al. Clinicopathological properties of poorly-differentiated adenocarcinoma of the stomach: comparison of solid- and non-solid-types. Anticancer Res 2006;26:639-646.

21. Kang SH, Moon HS, Sung JK, et al. Endoscopic prediction of tumor invasion depth in early gastric signet ring cell carcinoma. Dig Dis 2019;37:201-207.


- TOOLS
-
METRICS

-
- 2 Crossref
- 5,037 View
- 81 Download
- Related articles in Korean J Helicobacter Up Gastrointest Res
-
Considerations for Endoscopic Treatment of Undifferentiated-type Early Gastric Cancer2021 June;21(2)
Endoscopic Treatment of Submucosal Tumor from Upper Gastrointestinal Tract2011 September;11(2)