 |
 |
- Search
| Korean J Helicobacter Up Gastrointest Res > Volume 23(2); 2023 > Article |
|
Abstract
Autoimmune gastritis (AIG), a chronic inflammatory disease occurs as a result of a complex interaction between host-related and environmental factors. AIG may progress to severe atrophic gastritis secondary autoimmune-mediated parietal cell destruction in the stomach. AIG can be diagnosed based on anti-parietal cell antibody tests and endoscopy, which reveals widespread gastric corpus atrophy in patients with low serum pepsinogen I levels, a low pepsinogen I/II ratio, and elevated serum gastrin levels on serological testing. Tissue biopsy findings, which include mucosal atrophy and lymphocytic infiltration of the lamina propria may be useful for diagnostic confirmation. Decreased gastric acid secretion causes hypergastrinemia and enterochromaffin-like (ECL) cell proliferation, which can lead to neuroendocrine tumor development. Additionally, an autoimmune response results in parietal and chief cell injury, and proliferating ECL cells are detected in the deep mucosal layers in patients with AIG. Therefore, this condition may easily be misdiagnosed as a subepithelial tumor, and establishing a differential diagnosis for other types of subepithelial tumor accompanied by AIG is challenging. We present the case of a 54-year-old woman who was diagnosed with AIG with a concomitant subepithelial tumor based on serologic tests and biopsy findings and underwent wedge resection, which confirmed diagnosis of a schwannoma.
During embryonic development, the stomach develops from a gastric pouch divided into 2 anatomical and functional parts: the oxyntic mucosa, which forms the gastric body and fundus, and the mucous-secreting mucosa, which forms the antrum [1]. Atrophic gastritis is caused by chronic infiltration of inflammatory cells into the lamina propria, which destroys the mucosal glands, decreasing mucosal secretion in the stomach. The two most common causes are Helicobacter pylori infection and autoimmunity [1-3]. Autoimmune gastritis (AIG) is caused by an auto‑antibody targeting of the parietal cell’s H+/K+ ATPase. These complexes periodically destroy parietal cells via an auto-reactivated CD4+ T-cell-mediated immune response and activation of cytotoxic T cells. AIG leads to widespread atrophy of the gastric body and fundus[3]. Continuous destruction of parietal cells decreases secretion of gastric acid and intrinsic factors, which causes increased gastrin secretion from G cells in the antrum. This leads to hyperplasia of enterochromaffin-like (ECL) cells that secrete histamine in the gastric body or fundus, which further leads to neuroendocrine tumor (NET) development. ECL cells are located in the deep oxyntic mucosal area in patients with AIG; similarly to subepithelial tumors (SET) of NETs [3,4]. Although EUS is the best method to distinguish SETs and NETs, tissue biopsy may be required for a precise diagnosis, as well as additional endoscopic or surgical resection. Tumor resection or biopsy is often recommended for SETs measuring >2 cm. However, endoscopic resection is considered for tumors <2 cm classified as NETs or tumors with uncertain histological characteristics [5]. The occurrence of SETs in AIG accounts for 5% of neoplastic diseases occurring in the gastric mucosa and management has not yet been established [6]. Here, we report the case of a patient diagnosed with AIG after a serologic test, endoscopy, and biopsy of a <2 cm fixed-form SET in the lower body of the stomach that required wedge resection for accurate diagnosis and treatment, which confirmed it to be schwannoma. This case was approved by the Institutional Review Board of Wonkwang university hospital (No. WKUH 2023-03-012). Informed consent for it was obtained from patient for publication of this study.
A 54-year-old female patient presented at our hospital with a chief complaint of indigestion after meals for 2 years intermittently. At the time, the patient had chronic gastritis resulting from a previous hospital endoscopy, but had not undergone further testing and no previous endoscopic images were available. The patient was not treated for H. pylori infection, did not take any medications, including antacids and antibiotics, and had no underlying diseases for which she was undergoing regular follow-up examinations. We performed an upper gastrointestinal (GI) endoscopy, which revealed erythematous nodules, mucosal fold flattening, and atrophy on the greater curvature of the gastric body. However, there was no significant atrophy at the antrum area (Fig. 1). Serologic test results were pepsinogen Ⅰ level, 22.4 ng/mL; pepsinogen Ⅱ level, 13.2 ng/mL; and pepsinogen Ⅰ/Ⅱ ratio, 1.7. We found that serum gastrin level was high (205 pg/mL; reference value: 13~115 pg/mL), and that the anti-Helicobacter pylori antibody level was normal (0.73 U/mL; reference value: 0.0~0.89 U/mL). Upper GI endoscopy revealed atrophic gastritis in the gastric body and fundus, and a rapid urease test for H. pylori infection was negative. Hence, immunofluorescence analysis for anti-parietal cell antibodies was performed (Kallestad Mouse Stomach/Kidney; Bio-RAD, Hercules, CA, USA), which was positive. Biopsies were collected from the greater curvature of the gastric body and antrum. Notably, biopsy was performed on the slightly elevated erythematous nodule and the atrophic lesion at the greater curvature of the gastric body (Fig. 2A). From the antrum biopsy findings, the mucosa was found to be normal with no significant infiltration of inflammatory cells. However, biopsy performed in the body area showed inflammatory cell infiltration in the mucosal layer, confirming the AIG diagnosis. Inflammatory cell infiltration was significantly higher in the atrophic lesion than erythematous nodular lesion in the gastric body (Fig. 2B, C). Immunohistochemistry with chromogranin A was performed to compare ECL cells in the erythematous nodule area and the atrophic lesion in the gastric body; atrophic lesion had increased linear ECL cell hyperplasia compared to the erythematous nodular lesion (Fig. 2D, E). Upper GI endoscopy revealed a SET located at the greater curvature of the gastric lower body. The tumor measured slightly larger than 1 cm, did not show any rolling or cushion signs, and had a fixed shape (Fig. 3A). An abdominal computed tomography scan was performed to evaluate the SET size and location, which measured 1.2 cm and showed exophytic lesion in the gastric body (Fig. 3B). EUS showed a 1.1×0.8 cm oval tumor beneath the muscularis propria (Fig. 3C). The patient wanted the lesion excised and a wedge resection was performed for treatment and biopsy. The tumor’s dimensions and weight were 1.5×1 cm and 6 g, respectively, and it had a smooth surface. The inner surface of the tumor was gray, and biopsy findings revealed interlacing bundles of spindle cells (Fig. 3D). Simultaneously, immunohistochemical staining for S-100 protein was performed, and schwannoma was diagnosed with the positive findings (Fig. 3E). One month postoperatively, the patient no longer complained of GI symptoms.
AIG is characterized by atrophic changes in the gastric body and a normal mucous membrane in the antrum. However, the remaining parietal cells can appear as various oxyntic mucosal lesions in the gastric body, such as flat, elevated, pseudopolypoid, island, extensive, and granular lesions. Pathological changes can lead to H. pylori infection or bile acid regurgitation in the gastric antrum, such as patchy redness, circular wrinkle-like pattern, and red streak; likely to be missed on upper GI endoscopies. Mucosal changes may not be obvious in early stages of AIG, making a definitive diagnosis difficult unless gastric body and antrum biopsies and a serologic test are performed [7]. Slightly elevated erythematous nodules found in the gastric body on an upper GI endoscopy may be a characteristic feature of early-stage AIG. When erythematous and atrophic mucosa biopsies are performed, damage to the parietal and chief cells is greater in the atrophic mucosa than in the erythematous mucosa [8]. In the present case, erythematous nodules and atrophic mucosa were observed together in the gastric body, but inflammatory cell infiltration and ECL cell hyperplasia were more severe in the atrophic mucosa than in erythematous nodules. Serologic testing revealed low pepsinogen Ⅰ and pepsinogen Ⅰ/Ⅱ ratio and high serum gastrin levels, however pepsinogen I was higher and gastrin levels were lower than those observed in AIG patients [9]. Thus, this case is an example of an ongoing AIG stage, not early or advanced atrophy stage. AIG is a progressive inflammatory disease that can appear as various features on endoscopy depending on its stage, and show ambiguous serologic results, thus biopsy should be performed even if widespread atrophy is not apparent, and remnant oxyntic mucosal lesions mixed with atrophic mucosa and flattened folds are observed in the gastric body [10].
Asymptomatic gastric SETs <2 cm without ulceration, bleeding, or with increasing size can be followed up regularly by EUS. However, biopsy should not be avoided solely based on tumor size but should be considered when the mass arises from the muscularis propria or suspected of malignancy [11]. ECL cells are located deeper in the mucosa and infiltrate the submucosal layer in AIG. Although NETs from ECL cells did not occur in the proper muscle layer in this patient, they may resemble SETs, and endoscopic resection is recommended even if they are <1 cm to avoid the risk of lymph node metastasis [12]. Therefore, NETs accompanied by AIG may appear as SETs, occur in various locations, and may hide malignancy when they are <2 cm [13-17]. Table 1 summarizes cases reporting SETs in AIG patients thus far. A schwannoma, confirmed by biopsy, is a benign, asymptomatic tumor that can be discovered incidentally and has a good prognosis. Endoscopic resection is effective in some cases; however, it is not easy to perform. Hence, surgical resection remains the primary treatment option [18]. In the present case, the SET measured <2 cm, and EUS and computed tomography confirmed that the tumor, which was accompanied by AIG, was fixed, and located under the muscularis propria. Even though 60% of tumors originating from the proper muscle layer are GI stromal tumors, the EUS in this case showed a hypoechoic and homogenous tumor, consistent with a potential schwannoma diagnosis [5]. In the case of SETs <2 cm originating from the proper muscle layer, mucosal incision-assisted biopsy, EUS guided-fine needle biopsy or tumor resection to avoid unnecessary follow up may be an option in case of unknown histology [19]. Moreover, as the patient wanted the tumor excised, a wedge resection was performed to facilitate treatment and establish an accurate histological differential diagnosis of SETs originating from the muscularis propria such as schwannoma, leiomyoma or GI stromal tumor [5,19].
Hypergastrinemia resulting from the hypochlorhydria environment may contribute to ECL cell proliferation from hyperplasia to NETs [20]. In our case, as shown in Table 1, gastrin levels were not high compared to other cases; however, it is not yet known what effect hypergastrinemia has on SET development in AIG. There are no definitive guidelines for assessment and treatment of SETs in patients with AIG. Moreover, the correlation between these tumors and AIG is quite ambiguous; a schwannoma found in a SET may not be related to AIG. As SETs can manifest in various forms in patients with AIG, a systematic follow-up of similar cases, such as this one, is required.
Fig. 1.
Upper gastrointestinal endoscopy findings. (A) Erythematous nodules, flattened mucosal folds, and atrophic mucosa are observed in the gastric body and fundus. (B) Flattened gastric mucosal folds and prominent submucosal vasculature are observed along the greater curvature of the gastric body. (C) The gastric antrum appears normal.
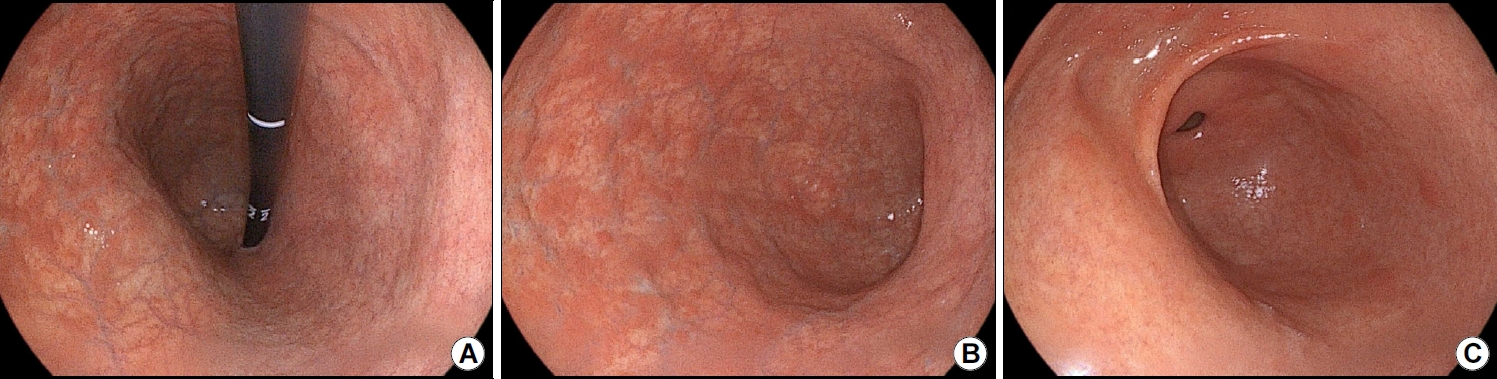
Fig. 2.
Histopathological findings of the greater curvature of the gastric body. (A) Endoscopic images showing slightly elevated erythematous nodules (blue arrow) and advanced mucosal atrophy (yellow arrow) along the greater curvature of the gastric mid body. (B) Specimen obtained from an erythematous nodule showing mild inflammation and atrophic oxyntic gastric mucosa (H&E stain, ×100). (C) Specimen obtained from the mucosa in an area of advanced atrophy showing moderate inflammation and atrophic oxyntic gastric mucosa (H&E stain, ×100). (D) Image of the same specimen as that shown in (B); mildly increased numbers of neuroendocrine cells are observed (chromogranin A stain, ×100). (E) Image of the same specimen as that shown in (C); a mild-to-moderate increase in the numbers of neuroendocrine cells and linear neuroendocrine cell hyperplasia is visualized (chromogranin A stain, ×100).
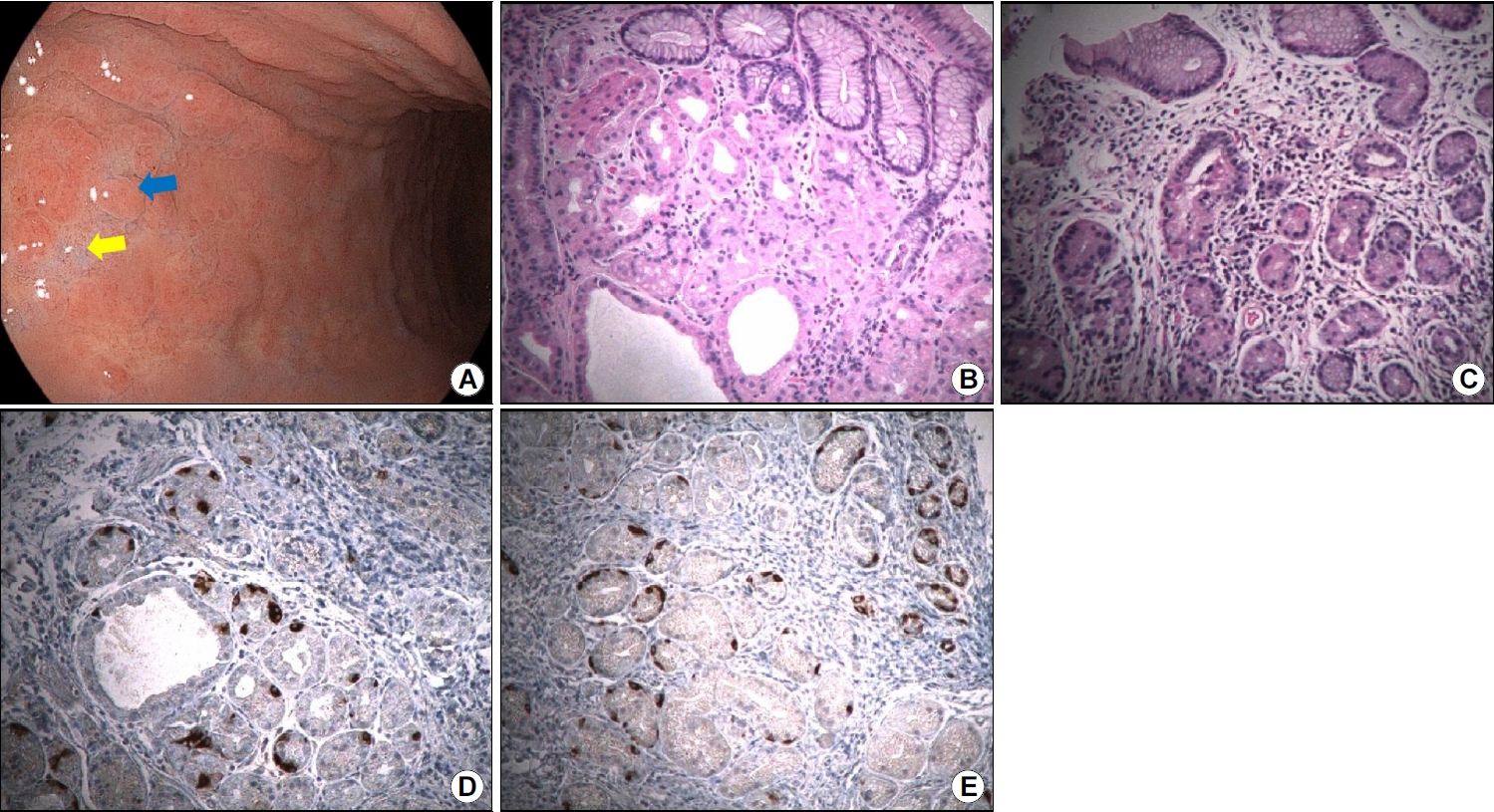
Fig. 3.
Images of the subepithelial tumor. (A) Endoscopic images showing a subepithelial tumor (slightly >1 cm) at the greater curvature of the gastric lower body. (B) Contrast-enhanced computed tomography scan showing an enhanced exophytic mass (1.2 cm) in the gastric body. (C) Endoscopic ultrasonography scan showing an oval hypoechoic mass (1.1×0.8 cm) with a clearly demarcated border, beneath the muscularis propria layer. (D) Specimen showing interlacing bundles of spindle cells with elongated and palisading nuclei (hematoxylin & eosin stain, ×100). (E) Specimen showing stained cytoplasm and nuclei of tumor cells (S-100 protein stain, ×100).
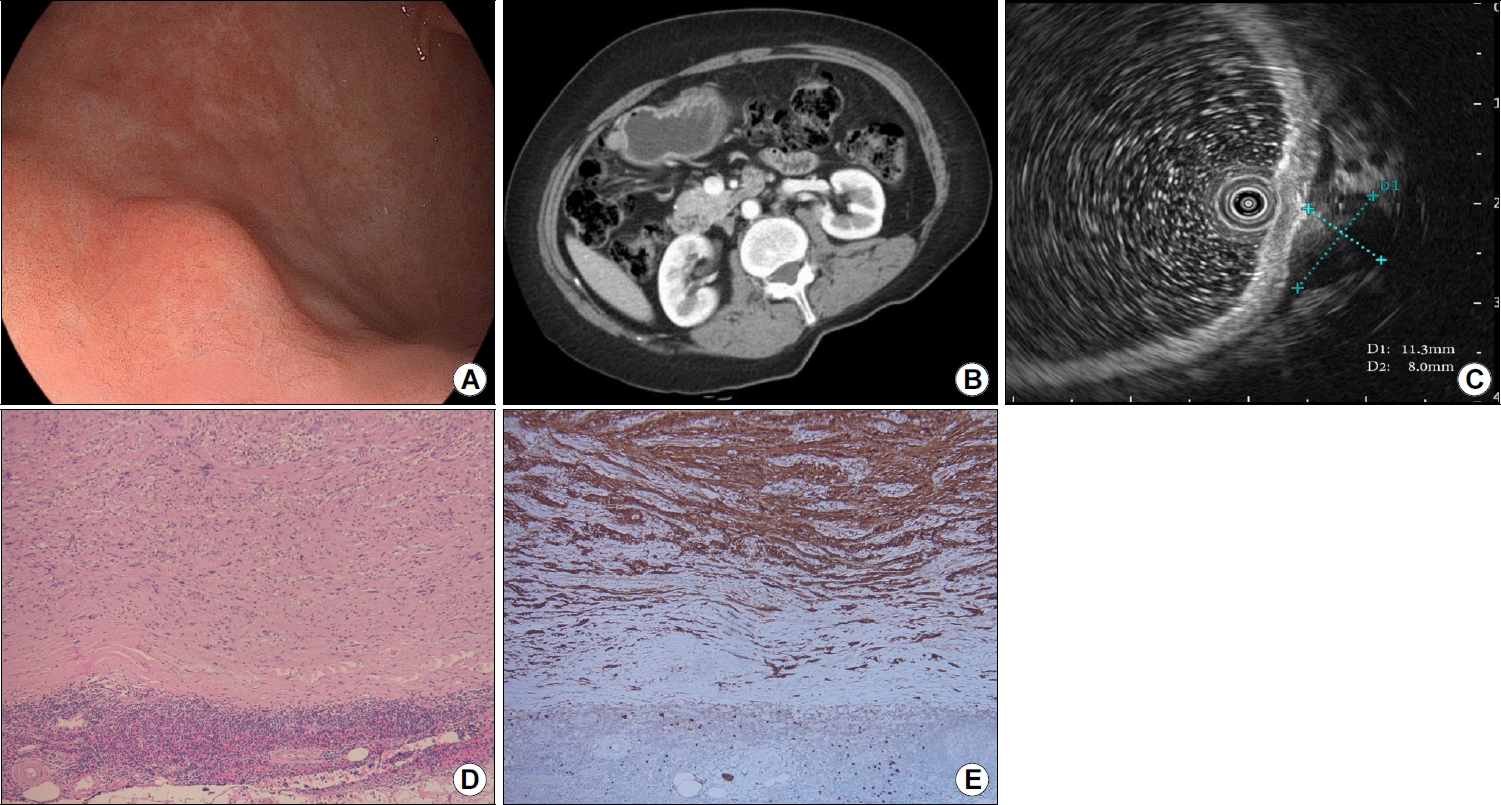
Table 1.
Reported Cases of Autoimmune Gastritis with Concomitant Subepithelial Tumors
| Authors | Age/sex | Anti-parietal cell antibody | Gastrin (pg/mL) | Size (mm) | Location and depth | Histologic diagnosis | Management | Others |
|---|---|---|---|---|---|---|---|---|
| Igarashi et al. [13] | 80/M | Negative | >3,000 | 6 | Greater curvature of the body, submucosa | Type 1 neuroendocrine tumor | LECS | Hyperchromic macrocytic anemia |
| Kubo et al. [14] | 71/M | Positive (AIFA positive) | 1,440 | 8 | Posterior wall of the gastric angle, submucosa | Type 1 neuroendocrine tumor | ESD | Synchronous adenocarcinoma (6×4 mm) |
| Mori et al. [15] | 60/F | Positive | 2,870 | 12 | Anterior wall of the middle body, submucosa | MiNENs | ESD | Consisting of adenocarcinoma and type 2 neuroendocrine tumor |
| Guerini et al. [16] | 50/F | Positive | 3,122 | 30 | Cardia, lamina propria | Pyloric gland adenoma | Proximal gastrectomy | Fundus ECL cell hyperplasia and dysplasia |
| Iwatsubo et al. [17] | 86/F | Negative (AIFA positive) | 4,900 | <10 | Greater curvature of the lower body, submucosa | Fundic gland type of adenocarcinoma | ESD | |
| Present case | 54/F | Positive | 205 | 15 | Greater curvature of the lower body, muscularis propria | Schwannoma | Wedge resection |
REFERENCES
1. Rugge M, Savarino E, Sbaraglia M, Bricca L, Malfertheiner P. Gastritis: the clinico-pathological spectrum. Dig Liver Dis 2021;53:1237–1246.


2. Sugano K, Tack J, Kuipers EJ, et al.; faculty members of Kyoto Global Consensus Conference. Kyoto global consensus report on Helicobacter pylori gastritis. Gut 2015;64:1353–1367.



4. Nehme F, Rowe K, Palko W, Tofteland N, Salyers W. Autoimmune metaplastic atrophic gastritis and association with neuroendocrine tumors of the stomach. Clin J Gastroenterol 2020;13:299–307.



5. Deprez PH, Moons LMG, OʼToole D, et al. Endoscopic management of subepithelial lesions including neuroendocrine neoplasms: European Society of Gastrointestinal Endoscopy (ESGE) Guideline. Endoscopy 2022;54:412–429.


6. Zhang H, Jin Z, Cui R, Ding S, Huang Y, Zhou L. Autoimmune metaplastic atrophic gastritis in chinese: a study of 320 patients at a large tertiary medical center. Scand J Gastroenterol 2017;52:150–156.


7. Kishino M, Nonaka K. Endoscopic features of autoimmune gastritis: focus on typical images and early images. J Clin Med 2022;11:3523.



8. Kishino M, Yao K, Hashimoto H, et al. A case of early autoimmune gastritis with characteristic endoscopic findings. Clin J Gastroenterol 2021;14:718–724.




9. Kishikawa H, Nakamura K, Ojiro K, et al. Relevance of pepsinogen, gastrin, and endoscopic atrophy in the diagnosis of autoimmune gastritis. Sci Rep 2022;12:4202.




10. Rustgi SD, Bijlani P, Shah SC. Autoimmune gastritis, with or without pernicious anemia: epidemiology, risk factors, and clinical management. Therap Adv Gastroenterol 2021;14:17562848211038771.




11. Sharzehi K, Sethi A, Savides T. AGA clinical practice update on management of subepithelial lesions encountered during routine endoscopy: expert review. Clin Gastroenterol Hepatol 2022;20:2435–2443; e4.


12. Namikawa K, Kamada T, Fujisaki J.; Collaborators. Clinical characteristics and long-term prognosis of type 1 gastric neuroendocrine tumors in a large Japanese national cohort. Dig Endosc 2023;Jan 31 [Epub]. https://doi.org/10.1111/den.14529.


13. Igarashi R, Irisawa A, Shibukawa G, et al. Case report of a small gastric neuroendocrine tumor in a deep layer of submucosa with diagnosis by endoscopic ultrasound-guided fine-needle aspiration and treatment with laparoscopic and endoscopic cooperative surgery. Clin Med Insights Case Rep 2018;11:1179–547617749226.




14. Kubo K, Kimura N, Matsuda S, Mabe K, Kato M. Synchronous early gastric cancer/neuroendocrine tumor associated with autoimmune gastritis completely resected with endoscopic submucosal dissection. Intern Med 2019;58:2633–2637.



15. Mori N, Hongo M, Takemura S, et al. Mixed neuroendocrine-non-neuroendocrine neoplasm associated with autoimmune gastritis. Clin Case Rep 2022;10:e05640.




16. Guerini C, Lenti MV, Rossi C, et al. Case report: two is not (always) better than one: pyloric gland adenoma of the gastric cardia and concurrent neuroendocrine cell dysplasia arising from autoimmune gastritis. Front Med (Lausanne) 2022;9:890794.



17. Iwatsubo T, Ota K, Takeuchi T. Submucosal tumor-like lesion in autoimmune gastritis: a rare case of fundic gland type of gastric adenocarcinoma. Clin Gastroenterol Hepatol 2023;21:A19.

18. Singh A, Aggarwal M, Chadalavada P, et al. Natural history of gastrointestinal schwannomas. Endosc Int Open 2022;10:E801–E808.



-
METRICS

-
- 0 Crossref
- 1,411 View
- 38 Download
- Related articles in Korean J Helicobacter Up Gastrointest Res
-
A Case of Early Gastric Adenocarcinoma Resembling Subepithelial Tumor2013 March;13(1)
A Case of Gastric Calcifying Fibrous Tumor Presenting as a Subepithelial Tumor2013 December;13(4)
Esophageal Squamous Cell Carcinoma Presenting as a Subepithelial Tumor2017 September;17(3)






