 |
 |
- Search
| Korean J Helicobacter Up Gastrointest Res > Volume 22(2); 2022 > Article |
|
Abstract
Conventional endoscopic mucosal resection (EMR) and endoscopic submucosal dissection (ESD) have been reported to be effective therapeutic options in sporadic non-ampullary duodenal tumors, but the rate of perforation is higher than that of other gastrointestinal lesions. Underwater EMR (UW EMR) has been reported to be a safe alternative to conventional EMR and ESD for superficial non-ampullary duodenal adenomas. We reviewed the medical chart and endoscopic report of patients who underwent endoscopic resection between August 2018 and February 2020. A total of 12 duodenal tumors were resected by UW EMR. The mean specimen and lesion sizes were 6.7 mm (2~16 mm) and 5.3 mm (2~10 mm), respectively. Of the 12 lesions, nine (75.0%) were located in the 2nd portion, and three (25.0%) were in the bulb. The mean procedural time was 7.8 minutes (3.2~18.7 minutes). Histologic results showed 10 dysplasia (nine low-grade, one high-grade) and two neuroendocrine tumors. UW EMR showed favorable efficacy and safety within small dysplastic lesions compared to previous studies’ results. Furthermore, it might be considered a treatment option with caution in patients with the duodenal subepithelial tumor within the third layer.
A superficial, non-ampullary duodenal epithelial tumor is considered a very rare disease with an incidence of only 0.3~4.6% [1-6]. Duodenal adenomas are precancerous lesions that have a risk of malignant transformation [7-9]. In recent years, the opportunity to detect duodenal lesions has increased with the development of endoscopy technologies and the popularization of endoscopic screening for upper gastrointestinal tract tumors [10-12].
For sporadic non-ampullary duodenal tumors, endoscopic resection using conventional endoscopic mucosal resection (EMR) and endoscopic submucosal dissection (ESD) were generally considered effective therapeutic options [12,13]. However, when removing duodenal lesions, the rate of perforation of EMR or ESD is higher than that of endoscopic resection for other gastrointestinal lesions [12]. Because the duodenal muscularis propria is extremely thin [12]. Injections of a solution into the submucosal layer during EMR increase the distance of lesions from the proper muscle layer to avoid perforation [3,6,14-17]. However, submucosal injections in the duodenum, where maneuverability is limited, make snaring of lesions more difficult [18]. In addition, there is a risk that neoplastic cells can be seeded into deeper duodenal wall layers along the needle tract during the submucosal injection [18].
In order to overcome the disadvantages of conventional EMR, an underwater EMR (UW EMR) using a buoyant force from filling the duodenal lumen with water has been developed instead of submucosal injection [18]. UW EMR, which removes superficial adenoma in the duodenum or in the large intestine with thin walls, has a very low perforation risk and can safely and completely remove that lesion [12]. Herein, we tried to evaluate the efficacy and safety of UW EMR for duodenal tumors that include the adenoma and the 3rd layer subepithelial tumors (SETs).
From August 2018 to February 2020, after medical chart and endoscopic report review, a total of 12 duodenal tumors resected by UW EMR were included. We evaluated the tumor location, pathology, length of procedure time, complete resection rate, incidence of complications, recurrence rates, and follow-up months. This work was reviewed and approved by the Institutional Review Board of the School of Medicine at Pusan National University (IRB No. 05-2020-164). Because of the retrospective nature, the requirement of informed consent was waived. All patients provided informed consent for the endoscopic procedures. It was conducted in accordance with the human and ethical principles of research specified by the Declaration of Helsinki.
The endoscope used for UW EMR was GIF-2TQ260M (Olympus Co., Tokyo, Japan). Depending on the size of the duodenal tumor, 10 mm or 15 mm polypectomy snare (Endo-Flex, Niederrhein, Germany) was selected according to the judgment of the two endoscopists. A high-frequency generator (VIO 300D; ERBE, Tuebingen, Germany) was used for the UW EMR procedure. Two endoscopists (S.J.K., D.G.R.) performed UW EMR for 12 patients during the 13-month period. All endoscopic procedures were performed under conscious sedation, unless contraindicated for a particular case. Midazolam was used for sedation and the initial dose was determined to be 0.15 to 0.30 mg/kg injected intravenously. The dose was increased or decreased depending on the patient's age and underlying disease. The posture of all patients was in the left lateral decubitus position. Warm distilled water was injected into the duodenal lumen through the accessory channel (Fig. 1A). The injection of water was continued until the tumor was completely immersed in distilled water. In the underwater immersion state, after confirming that the target tumor was slightly bulging from the mucosal surface, a snare was inserted through another endoscopic channel. EUS was performed to evaluate the origin of tumor (Fig. 1B). Subsequently, the duodenal tumor was excised using an incision wave (Fig. 1C-E). Preoperative marking has not been performed, because the boundary between the tumor and the normal mucosal surface in water became morphologically clear during UW EMR. If there was a remnant tumor after UW EMR, an additional resection was performed. If there was bleeding after resection, hemostasis was performed using thermal coagulation. Clipping was not performed since the lesion was small. If no specific adverse events were observed, postoperative diet was started at the day after the procedure. To evaluate local recurrence at the resection site, periodic follow-up endoscopic examinations were underwent for all of the patients. The total procedure time was counted from the initiation of water infusion to the removal of the tumor. We also assessed the occurrence of adverse events including bleeding and perforation. The first endoscopic evaluation with biopsy was performed approximately 3 months after the endoscopic resection.
The median age of the patients was 52.8 years (range, 36~68). The mean specimen size was 6.7 mm (range, 2.0~16.0) and the mean lesion size was 5.3 mm (range, 2.0~10.0). Nine lesions (75.0%) were located in the 2nd portion of duodenum, and three lesions (25.0%) were in the bulb (Table 1). In all patients, en bloc resection was achieved. The mean procedural time was 7.8 minutes (range, 3.2~18.7). Histologic assessments of the removed specimens revealed nine adenomas with low-grade dysplasia (75.0%), one adenoma with high-grade dysplasia (8.33%) and two neuroendocrine tumors (NETs) (16.7%) (Fig. 1F). Both NET cases were G1 grade. Evaluation of the margin of resected specimens showed one case with margin involvement (one of the NET cases), although the margin of resected specimen was grossly free. Follow-up endoscopic examinations were performed at 3 and 15 months after UW EMR. The follow-up endoscopic biopsy at the UW EMR site showed no recurrence in all cases (Table 2). Adverse events related to the procedure was occurred as minimal oozing bleeding at EMR site in one patient. The thermal coagulation achieved a successful hemostasis. There were no serious adverse events, such as perforation or aspiration pneumonia.
In our cases, UW EMR was useful for the removal of both duodenal epithelial tumors and SETs originating from 3rd layer. EMR of duodenal adenoma is generally difficult compared to colonoscopic polypectomy [18]. Because duodenum mucosa and submucosa are very thin and richly vascular [13,18]. Expanding the lumen with air during the conventional EMR makes the thin duodenal wall even thinner and, thus, increases the risk of perforation [18]. In addition, submucosal injection during EMR to reduce the risk of perforation lifts not only the lesion but also the surrounding mucosa. This results in the tissue tension surrounding adenoma that makes the snaring of lesion more difficult [18,19].
The water filling during UW EMR keeps the proper muscle layer circular in configuration, maintains native thickness during peristaltic contractions [18]. Furthermore, water immersion makes, duodenal tumors to float in the water, similar to a lifting sign after submucosal injection [18]. This effect is caused by the contraction of the superficial layers submerged in water, and is also caused by buoyancy generated by the lower density fatty tissue of the submucosa [18]. Another advantage of UW EMR is that the filling saline changes the duodenal lumen from winding to gently sloping [13]. Therefore, previous studies have reported that UW EMR is a safe procedure for resection of non-ampullary duodenal adenomas that does not cause adverse events, such as perforation [12,20,21]. According to a systematic review and meta-analysis of UW EMR performed on 258 recently published lesions, it was reported that the clinical success rate was 89.9% (95% CI, 83.4~94.1) and en bloc removal was achieved in 84.6% of treated lesions (95% CI, 75.5~90.7) [22]. In their study, the pooled rate of adverse events was reported as 6.9% (95% CI, 2.5~17.9), but most of them were reported as being self-limited delayed bleeding, and serious complications such as duodenal perforation were not reported [22]. In addition, UW EMR has a high success rate for the complete removal of large sessile duodenal adenomas [18].
UW EMR needed a short procedure time, although R0 resection rate was similar with ESD. Among the cases, a total of two cases of SET were removed by UW EMR. En bloc resection was achieved successfully in all cases. A notable strength is that the duodenal lesions resected by UW EMR included not only adenomas, but also such as NETs. Based on these results, UW EMR can be considered a treatment option for 3rd layer origin duodenal submucosal tumors including NETs. To make definitive conclusions about the effectiveness of UW EMR for the removal of duodenal NETs requires more cases outcomes.
In conclusion, UW EMR showed favorable efficacy and safety for small dysplastic lesions. Therefore, it might be considered as an option of treatment modality for duodenal SETs including NETs. Although we found that UW EMR can safely remove duodena NETs, a large-sized, prospective multi-center, controlled trial is required.
Acknowledgements
This work was supported by a 2022 research grant from Pusan National University Yangsan Hospital.
We declare that all the listed authors have participated actively and all met the requirements of the authorship. All authors have read and approved the manuscript. Dr. JWL, Dr. SJK designed the work, wrote the protocol and performed the procedure. Dr. JWL, Dr. SJK and Dr. DGR assisted in the operation and collected the clinical data. Dr. JWL Dr. SJK and Dr. CWC managed the literature searches. Dr. JWL undertook the statistical analysis. Dr. JWL wrote the first draft of the manuscript.
Fig. 1.
Underwater endoscopic mucosal resection procedure of a duodenal tumor. Endoscopic image showing a 6×4 mm, yellowish, hard subepithelial tumor with central depression in the 2nd portion of the duodenum (A). Endoscopic ultrasonography revealing a 7×3 mm, homogeneous, hypoechoic solid lesion in the third layer (B). Water filling makes it easier for an endoscopist to snare the tumor (C). There are no residual lesions (D). En bloc resection achieved (E). The histological type of the resected subepithelial tumor is a neuroendocrine tumor of grade G1 (mitotic rate: 0/10 high power field, Ki67 proliferation index: 1%; synaptophysin, ×100) (F).
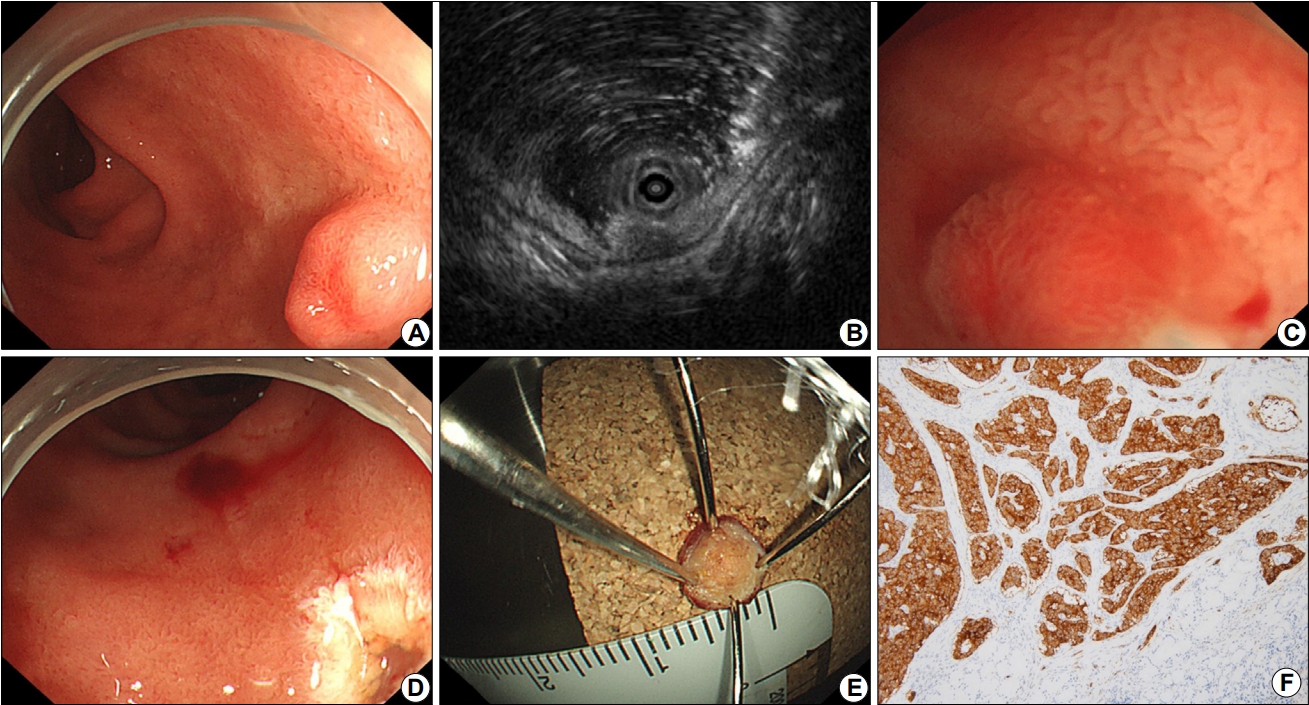
Table 1.
Patient and Treatment Characteristics
Table 2.
Clinical Outcomes
REFERENCES
1. Murray MA, Zimmerman MJ, Ee HC. Sporadic duodenal adenoma is associated with colorectal neoplasia. Gut 2004;53:261–265.



2. Arai T, Murata T, Sawabe M, Takubo K, Esaki Y. Primary adenocarcinoma of the duodenum in the elderly: clinicopathological and immunohistochemical study of 17 cases. Pathol Int 1999;49:23–29.



3. Abbass R, Rigaux J, Al-Kawas FH. Nonampullary duodenal polyps: characteristics and endoscopic management. Gastrointest Endosc 2010;71:754–759.


4. Jepsen JM, Persson M, Jakobsen NO, et al. Prospective study of prevalence and endoscopic and histopathologic characteristics of duodenal polyps in patients submitted to upper endoscopy. Scand J Gastroenterol 1994;29:483–487.


5. Miller JH, Gisvold JJ, Weiland LH, McIlrath DC. Upper gastrointestinal tract: villous tumors. AJR Am J Roentgenol 1980;134:933–936.


6. Yamamoto Y, Yoshizawa N, Tomida H, Fujisaki J, Igarashi M. Therapeutic outcomes of endoscopic resection for superficial non-ampullary duodenal tumor. Dig Endosc 2014;26 Suppl 2:50–56.


7. Galandiuk S, Hermann RE, Jagelman DG, Fazio VW, Sivak MV. Villous tumors of the duodenum. Ann Surg 1988;207:234–239.



8. Perzin KH, Bridge MF. Adenomas of the small intestine: a clinicopathologic review of 51 cases and a study of their relationship to carcinoma. Cancer 1981;48:799–819.


9. Sellner F. Investigations on the significance of the adenoma-carcinoma sequence in the small bowel. Cancer 1990;66:702–715.


10. Goda K, Kikuchi D, Yamamoto Y, et al. Endoscopic diagnosis of superficial non-ampullary duodenal epithelial tumors in Japan: Multicenter case series. Dig Endosc 2014;26 Suppl 2:23–29.


11. Kakushima N, Ono H, Takao T, Kanemoto H, Sasaki K. Method and timing of resection of superficial non-ampullary duodenal epithelial tumors. Dig Endosc 2014;26 Suppl 2:35–40.


12. Shibukawa G, Irisawa A, Sato A, et al. Endoscopic mucosal resection performed underwater for non-ampullary duodenal epithelial tumor: evaluation of feasibility and safety. Gastroenterol Res Pract 2018;2018:7490961.




13. Kiguchi Y, Kato M, Nakayama A, et al. Feasibility study comparing underwater endoscopic mucosal resection and conventional endoscopic mucosal resection for superficial non-ampullary duodenal epithelial tumor < 20 mm. Dig Endosc 2020;32:753–760.



14. Yahagi N, Kato M, Ochiai Y, et al. Outcomes of endoscopic resection for superficial duodenal epithelial neoplasia. Gastrointest Endosc 2018;88:676–682.


15. Apel D, Jakobs R, Spiethoff A, Riemann JF. Follow-up after endoscopic snare resection of duodenal adenomas. Endoscopy 2005;37:444–448.


16. Lépilliez V, Chemaly M, Ponchon T, Napoleon B, Saurin JC. Endoscopic resection of sporadic duodenal adenomas: an efficient technique with a substantial risk of delayed bleeding. Endoscopy 2008;40:806–810.


17. Alexander S, Bourke MJ, Williams SJ, Bailey A, Co J. EMR of large, sessile, sporadic nonampullary duodenal adenomas: technical aspects and long-term outcome (with videos). Gastrointest Endosc 2009;69:66–73.


18. Binmoeller KF, Shah JN, Bhat YM, Kane SD. "Underwater" EMR of sporadic laterally spreading nonampullary duodenal adenomas (with video). Gastrointest Endosc 2013;78:496–502.


19. ASGE TECHNOLOGY COMMITTEE, Kantsevoy SV, Adler DG, et al. Endoscopic mucosal resection and endoscopic submucosal dissection. Gastrointest Endosc 2008;68:11–18.


20. Yamasaki Y, Uedo N, Takeuchi Y, Ishihara R, Okada H, Iishi H. Current status of endoscopic resection for superficial nonampullary duodenal epithelial tumors. Digestion 2018;97:45–51.



-
METRICS

-
- 0 Crossref
- 2,268 View
- 74 Download
- Related articles in Korean J Helicobacter Up Gastrointest Res
-
Endoscopic Papillectomy for Ampullary Tumors2022 December;22(4)
Non-Ampullary Duodenal Tumors2022 December;22(4)
A Novel Knife for Endoscopic Submucosal Dissection in Early Gastric Cancer2022 March;22(1)
Endoscopic Resection of Gastrointestinal Stromal Tumor: Is It Safe?2021 September;21(3)
Clinical Practice Guideline for Endoscopic Resection of Early Gastrointestinal Cancer2020 June;20(2)






