 |
 |
- Search
| Korean J Helicobacter Up Gastrointest Res > Volume 23(4); 2023 > Article |
|
Abstract
Objectives
Methods
Results
Notes
Availability of Data and Material
The datasets generated or analyzed during the study are available from the corresponding author on reasonable request.
Authors’ Contribution
Conceptualization: Yuri Kim, Hwoon-Yong Jung. Data curation: all authors. Formal analysis: Yuri Kim. Investigation: Yuri Kim. Methodology: Hwoon-Yong Jung. Project administration: Hwoon-Yong Jung. Supervision: Hwoon-Yong Jung. Validation: all authors. Visualization: Yuri Kim. Writing—original draft: Yuri Kim. Writing—review & editing: Yuri Kim. Approval of final manuscript: all authors.
Fig. 1.

Fig. 2.
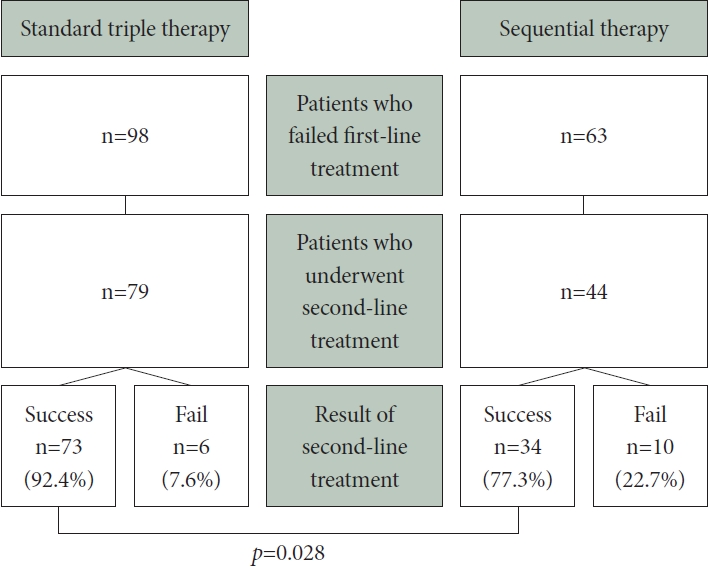
Fig. 3.
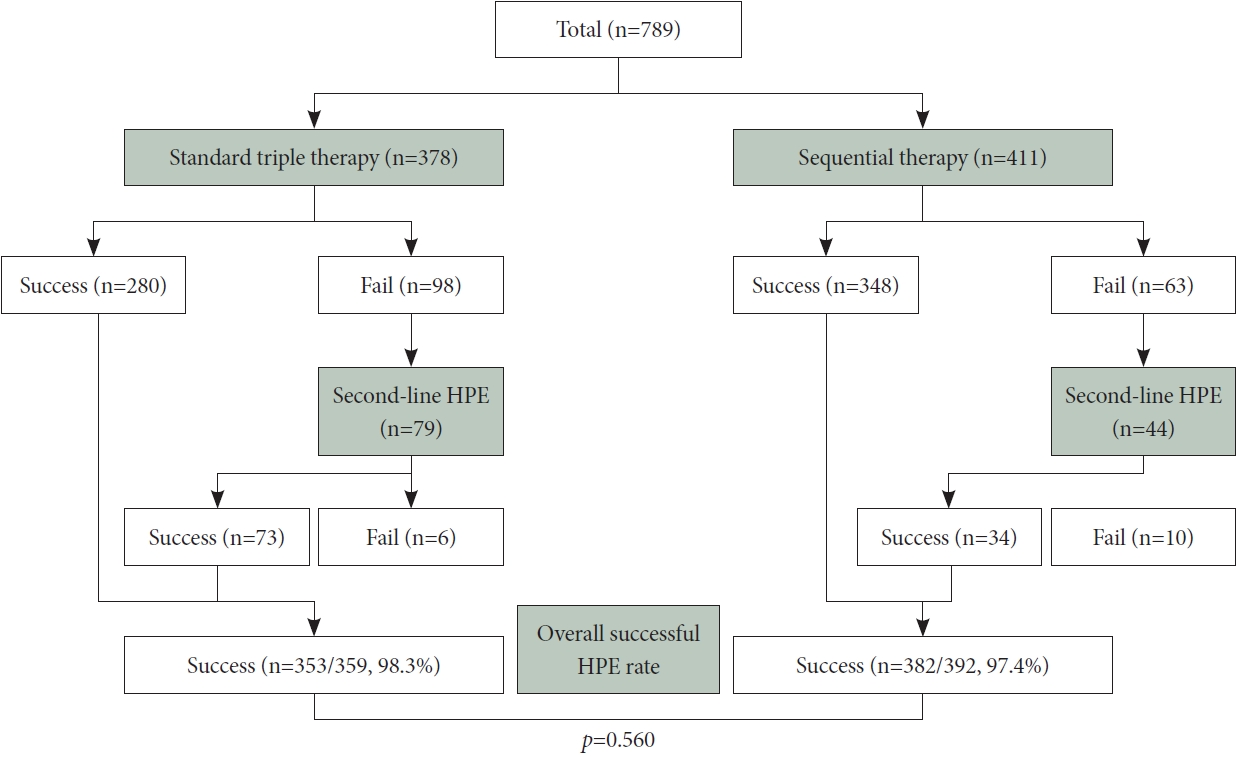
Table 1.
Table 2.
| Total (n=789) | Standard triple therapy (n=378) | Sequential therapy (n=411) | p-value | |
|---|---|---|---|---|
| Successful eradication | 628 (79.6) | 280 (74.1) | 348 (84.7) | <0.001 |
Table 3.
REFERENCES
-
METRICS

-
- 1 Crossref
- 1,500 View
- 45 Download
- Related articles in Korean J Helicobacter Up Gastrointest Res
-
What Is the Optimal Drug Regimen for Helicobacter pylori Eradication Therapy?2024 June;24(2)
Is Helicobacter pylori Infection Associated With Ulcerative Colitis Activity?2024 March;24(1)
Can Helicobacter pylori Infection Reduce the Efficacy of Cancer Immunotherapy?2023 December;23(4)






