 |
 |
- Search
| Korean J Helicobacter Up Gastrointest Res > Volume 21(4); 2021 > Article |
|

Abstract
Background/Aims
Materials and Methods
Results
Acknowledgements
Fig.┬Ā1.
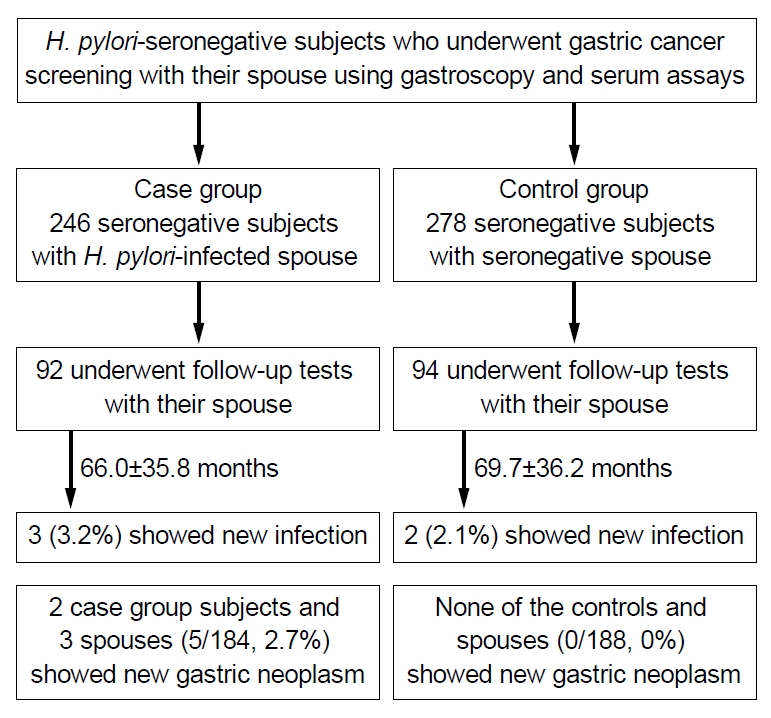
Fig.┬Ā2.
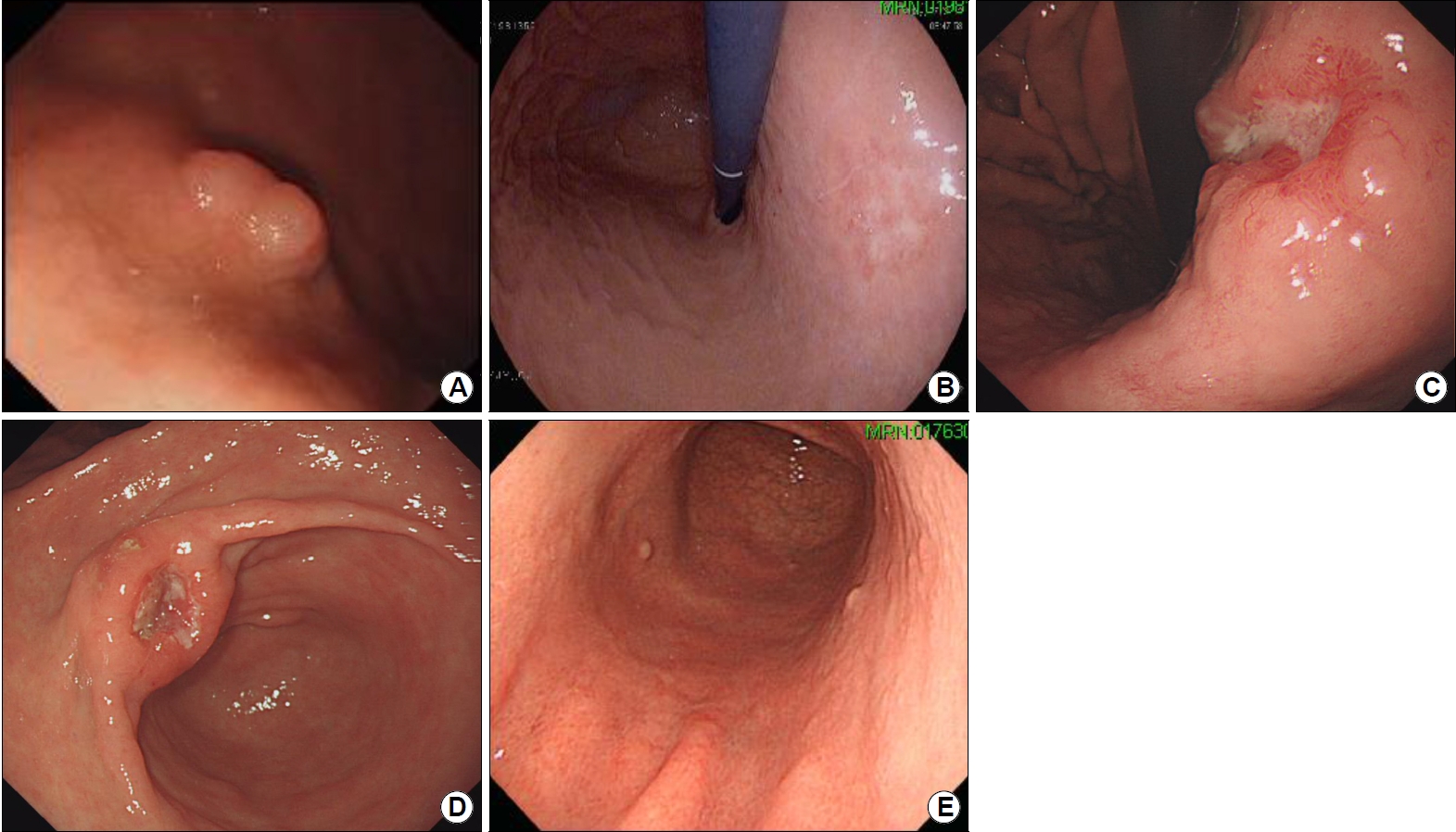
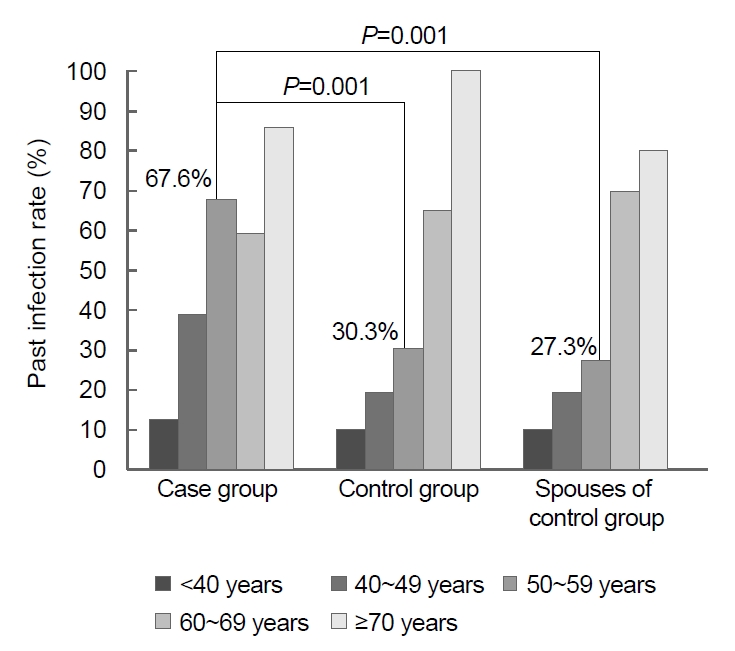
Fig.┬Ā3.
Table┬Ā1.
| Variable | Seronegative subjects with H. pylori-infected spouse (case group, n=92) | Seronegative subjects with seronegative spouse (control group, n=94) | P-value |
|---|---|---|---|
| Age | 54.6┬▒10.4 | 52.6┬▒10.9 | 0.197 |
| Male sex | 50 (54.3) | 49 (52.1) | 0.762 |
| Past H. pylori infection | 52 (56.5) | 34 (36.2) | 0.005 |
| Comorbidities | 21 (22.8) | 13 (13.8) | 0.112 |
| Diabetes mellitus | 3 (3.3) | 6 (6.4) | 0.497a |
| Hypertension | 20 (21.7) | 11 (11.7) | 0.066 |
| Antithrombotic agent | 5 (5.4) | 6 (6.4) | 0.784 |
| Nonsteroidal anti-inflammatory drug | 5 (5.4) | 1 (1.1) | 0.116a |
| Acid suppressant | 2 (2.2) | 0 | 0.243a |
| H. pylori serology test, Vidas:Chorus | 64:28 | 68:26 | 0.677 |
| Anti-H. pylori IgG, Vidas (AU/mL) | 0.50┬▒0.23 | 0.47┬▒0.23 | 0.432 |
| Anti-H. pylori IgG, Chorus, (AU/mL) | 6.5┬▒1.7 | 6.9┬▒1.8 | 0.486 |
| Serum PG I level (ng/mL) | 48.5┬▒18.3 | 49.5┬▒23.7 | 0.728 |
| Serum PG II level (ng/mL) | 9.3┬▒3.7 | 9.3┬▒5.8 | 0.979 |
| Serum PG I/II ratio | 5.4┬▒1.5 | 5.7┬▒1.5 | 0.180 |
| Total Kyoto classification score | 0.84 (0~4) | 0.46 (0~4) | 0.010 |
| Chronic atrophic gastritis score | 0.46 (0~2) | 0.24 (0~2) | 0.004 |
| Metaplastic gastritis score | 0.51 (0~2) | 0.32 (0~2) | 0.083 |
Values are presented as the mean┬▒standard deviation or number of the subjects with proportion (%) using the t-test and chi-square test for continuous and categorical variables. For continuous variables with asymmetrical distribution, data are presented as median with ranges using the Kruskal-Wallis test.
H. pylori, Helicobacter pylori; AU, arbitrary unit; PG, pepsinogen.
Table┬Ā2.
| Variable | Spouses of case group (n=92) | Spouses of control group (n=94) | P-value |
|---|---|---|---|
| Age | 55.2┬▒10.2 | 52.7┬▒11.2 | 0.109 |
| Male sex | 42 (45.7) | 45 (47.9) | 0.762 |
| Status of H. pylori infection, current:past:no | 92:0:0 | 0:33:61 | <0.001 |
| Comorbidities | 18 (19.6) | 12 (12.8) | 0.207 |
| Diabetes mellitus | 3 (3.3) | 6 (6.4) | 0.497a |
| Hypertension | 12 (13.0) | 10 (10.6) | 0.612 |
| Antithrombotic agent | 10 (10.9) | 5 (5.3) | 0.165 |
| Nonsteroidal anti-inflammatory drug | 3 (3.3) | 1 (1.1) | 0.366a |
| Acid suppressant | 2 (2.2) | 0 | 0.243a |
| H. pylori serology test, Vidas:Chorus | 64:28 | 68:26 | 0.677 |
| Anti-H. pylori IgG Vidas (AU/mL) | 3.45┬▒0.77 | 0.46┬▒0.23 | <0.001 |
| Anti-H. pylori IgG Chorus (AU/mL) | 150.7┬▒48.9 | 6.6┬▒1.8 | <0.001 |
| Serum PG I level (ng/mL) | 68.5┬▒33.3 | 48.5┬▒19.9 | <0.001 |
| Serum PG II level (ng/mL) | 20.9┬▒9.4 | 8.7┬▒3.4 | <0.001 |
| Serum PG I/II ratio | 3.4┬▒1.3 | 5.8┬▒1.4 | <0.001 |
| Total Kyoto classification score | 4.22 (2~7) | 0.46 (0~4) | <0.001 |
| Chronic atrophic gastritis score | 1.41 (0~2) | 0.33 (0~2) | <0.001 |
| Metaplastic gastritis score | 1.11 (0~2) | 0.32 (0~2) | <0.001 |
| Hypertrophic rugae score | 0.01 (0~1) | 0 | 0.313 |
| Nodular gastritis score | 0.02 (0~1) | 0 | 0.152 |
| Diffuse redness score | 1.66 (0~2) | 0 | <0.001 |
Values are presented as the mean┬▒standard deviation or number of the subjects with proportion (%) using the t-test and chi-square test for continuous and categorical variables. For continuous variables with asymmetrical distribution, data are presented as median with ranges using the Kruskal-Wallis test.
H. pylori, Helicobacter pylori; AU, arbitrary unit; PG, pepsinogen.
Table┬Ā3.
| Seronegative subject | Infection status (Kyoto scores) | Initial serum assay finding | Duration (months) | Follow-up test findings indicating new infection | Spouse | Infection status of spouse | Serum assay findings of spouse | |
|---|---|---|---|---|---|---|---|---|
| Case group | M/60 | Past (A2IM2H0N0DR0) | 0.35 AU/mL (Vidas) | 73 | 120.6 AU/mL (Chorus)Ōåæ | F/53 | Current (A2IM2H0N0DR0) | 3.31 AU/mL (Vidas) |
| PG I 47.8 ng/mL | PG I 116.5 ng/mLŌåæ | PG I 62.0 ng/mL | ||||||
| PG II 7.8 ng/mL | PG II 19.2 ng/mLŌåæ | PG II 13.7 ng/mL | ||||||
| PG I/II 6.1 | PG I/II 6.1 | PG I/II 4.5 | ||||||
| F/67 | Past (A2IM2H0N0DR0) | 0.65 AU/mL (Vidas) | 49 | >200 AU/mL (Chorus)Ōåæ | M/71a | Current (A2IM2H0N0DR0) | >4.0 AU/mL (Vidas) | |
| PG I 45.2 ng/mL | PG I 116.0 ng/mLŌåæ | PG I 10.3 ng/mL | ||||||
| PG II 10.5 ng/mL | PG II 35.6 ng/mLŌåæ | PG II 11.0 ng/mL | ||||||
| PG I/II 4.3 | PG I/II 3.3Ōåō | PG I/II 0.9 | ||||||
| M/51 | Past (A2IM2H0N0DR0) | 0.90 AU/mL (Vidas) | 12 | Positive Giemsa staining (no serum assay on the same day of endoscopy) | F/49 | Current infection (A2IM2H0N0DR0) | 2.61 AU/mL(Vidas) | |
| PG I 48.5 ng/mL | PG I 49.1 ng/mL | |||||||
| PG II 12.6 ng/mL | PG II 9.8 ng/mL | |||||||
| PG I/II 3.9 | PG I/II 5.0 | |||||||
| Control group | M/66 | No (A2IM2H0N0DR0) | 11.0 AU/mL (Chorus) | 67 | >200 AU/mL (Chorus)Ōåæ | F/63 | No (A2IM2H0N0DR0) | 5.9 AU/mL (Chorus) |
| PG I 40.8 ng/mL | PG I 60.1 ng/mLŌåæ | PG I 99.9 ng/mL | ||||||
| PG II 7.7 ng/mL | PG II 27.3 ng/mLŌåæ | G II 15.1 ng/mL | ||||||
| PG I/II 5.3 | PG I/II 2.2Ōåō | PG I/II 6.6 | ||||||
| M/43 | Past (A2IM2H0N0DR0) | 0.26 AU/mL (Vidas) | 96 | Positive Giemsa staining (no serum assay on the same day of endoscopy) | F/39 | No (A2IM2H0N0DR0) | 0.28 AU/mL (Vidas) | |
| PG I 52.1 ng/mL | PG I 48.4 ng/mL | |||||||
| PG II 8.1 ng/mL | PG II 5.1 ng/mL | |||||||
| PG I/II 6.5 | PG I/II 9.4 | |||||||
H. pylori, Helicobacter pylori; M, male; F, female; A2, open-type chronic atrophic gastritis; IM2, intestinal metaplasia extended to the corpus; H0, absence of hypertrophic rugae; N0, nodular gastritis absent; DR0, no diffuse or spotty redness; AU, arbitrary unit; PG, pepsinogen; Ōåæ, higher than the initial test finding; Ōåō, lower than the initial test finding.
Table┬Ā4.
M, male; F, female; A2, open-type chronic atrophic gastritis; IM2, intestinal metaplasia extended to the corpus; H0, absence of hypertrophic rugae; N0, nodular gastritis absent; DR0, no diffuse or spotty redness; A1, closed-type 2 or 3 CAG; DR2, severe redness; DR1, mild redness; H. pylori, Helicobacter pylori; AU, arbitrary unit; PG, pepsinogen; A0, closed-type 0 or 1 CAG; IM1, IM limited in the antrum; IM0, no IM.
Table┬Ā5.
REFERENCES
- TOOLS
-
METRICS

-
- 1 Crossref
- 6,176 View
- 65 Download
- Related articles in Korean J Helicobacter Up Gastrointest Res
-
Approach to Patients with Consecutive Helicobacter pylori Eradication Failure2023 March;23(1)
Is Probiotic Supplementation Useful for Helicobacter pylori Eradication?2023 March;23(1)






