 |
 |
- Search
| Korean J Helicobacter Up Gastrointest Res > Volume 23(2); 2023 > Article |
|
See editorial "Bismuth, An Indispensable Component of Helicobacter pylori Eradication Therapy" in Volume 23 on page 79.
Abstract
Background/Aims
Compared with other regimens, concomitant therapy (CT) used as a first-line regimen for Helicobacter pylori (H. pylori) infection is associated with higher eradication rates. We compared the efficacy of tailored therapy (TT) using bismuth added to standard triple therapy (STT) with CT.
Methods
This consecutive study performed between September 2020 and 2021 included 210 patients with H. pylori infection. Two participating gastroenterologists prescribed TT and CT. Multiplex PCR assays were performed before eradication therapy to identify the relevant point mutations and confirm clarithromycin resistance in the TT group (n=105). Patients who showed negative PCR results received 14-day STT and those with positive PCR results received a 14-day regimen of bismuth added to STT. The other group (n=105) received 10-day CT.
Results
Based on per-protocol analysis, eradication rates in the TT and CT groups were 89.2% (91/102) and 81.6% (84/103), respectively. We observed no statistically significant intergroup differences in eradication rates (P=0.12). The frequency of estimated clarithromycin resistance confirmed using multiplex PCR assays was 32.4% (34/105), and the eradication rate associated with bismuth add-on STT was 76.5% (26/34) in patients with clarithromycin resistance.
The clarithromycin resistance rate has increased tremendously, resulting in the failure of Helicobacter pylori (H. pylori) eradication. Clarithromycin-based triple therapy consists of a proton pump inhibitor (PPI), amoxicillin, and clarithromycin; however, it is no longer effective in regions with high clarithromycin resistance. The investigated clarithromycin resistance rates in the Korean population markedly vary with the area, ranging from 17% to 38.5% [1,2]. In some parts of Korea, clarithromycin-based standard triple therapy (STT) is still effective for H. pylori eradication and has been recommended as a first-line treatment. The empirical first-line regimen with STT was recommended only in areas with clarithromycin resistance rates of less than 15% due to the low eradication rate of STT [3]. Bismuth quadruple therapy (BQT) is suggested as the first-line treatment in areas with clarithromycin resistance rates above 15~20% [4]. No appropriate second-line regimen is available in case of eradication failure with first-line BQT. To date, a levofloxacin-based rescue regimen has been suggested as a candidate second-line regimen; however, because levofloxacin-resistant H. pylori strains are prevalent worldwide, such regimens may not be effective [5,6].
In a clinical trial, concomitant therapy (CT) resulted in optimal eradication of H. pylori among various treatment strategies. CT includes a PPI and concomitant use of three antibiotics: amoxicillin, metronidazole, and clarithromycin. Although the optimal treatment period is disputed, a treatment period of at least 10 days resulted in greater than 90% eradication. In Korea, several studies demonstrated that metronidazole-based triple therapy, CT, sequential therapy-CT resulted in the optimal eradication rate of 80~86% in intentional treat (ITT) analysis, representing alternative first-line regimens [7-9]. The major challenge associated with CT was patients’ poor compliance, which led to treatment failure. Also, antibiotic abuse is another major issue, and in case of eradication failure with CT, an appropriate second-line regimen is unavailable [7].
There is a growing interest in personalized healthcare, specifically regarding first-line eradication therapy, tailored therapy (TT) based on antibiotic susceptibility using nucleic acid-based techniques for identifying clarithromycin resistance-associated point mutations [10]. In the past, these nucleic acid-based techniques were expensive and labor-intensive, but recently they are popular and easily available. The treatment regimens indicated for clarithromycin resistance associated with point mutations are highly disputed [11,12]. Bismuth is not an antibiotic; however, adding bismuth to STT increased the efficacy against clarithromycin-resistant strains of H. pylori without increasing the risk of further antibiotic exposure [13,14]. The aim of this study was to compare the efficacy of TT based on bismuth add-on STT with CT as a first-line regimen for H. pylori eradication.
We conducted this consecutive study at the Catholic University School of Medicine, St. Vincent’s Hospital, from September 2020 to September 2021, and reviewed the chart retrospectively. We enrolled patients who underwent first-line eradication therapy. The diagnosis of H. pylori infection was based on two or more positive test results for H. pylori infection using rapid urease test (RUT), PCR, 13C-urea breath test (UBT), histologic evidence with silver stain, and serum IgG antibodies against H. pylori. Two biopsies were needed for RUT (CLO® test; Kimberly-Clark, Ogden, UT, USA) at the stomach antral and corpus mucosal tissues. We performed a 13C-urea breath test with a Pranactin®-Citric drug product, a component of the BreathTekTM UBT Kit (Korea Otsuka Pharmaceutical Co. Ltd., Seoul, Korea). The patients ingested 3 g of reconstituted Pranactin®-Citric containing 75 mg of 13C-urea. After mouthwash, we collected breath samples before and 20 minutes following the administration of 13C-urea. The 13C/12C ratio in the breath sample was measured with an infrared spectrophotometer (UBiT-IR300; Korea Otsuka Pharmaceutical Co. Ltd.). Changes in the 13C values over baseline were expressed as ∆13C, and a positive result was defined as an increase of more than 2.5%. The presence of serum Ig antibodies against H. pylori (Enzygnost®; Dade Behring, Marburg, Germany) was measured, and antibody titers over 15 U/mL were classified as H. pylori-seropositive by the manufacturer’s instructions.
Eligible subjects were older than 18 years, infected with gastric H. pylori confirmed with the tests, and without a history of H. pylori eradication. Subjects with a history of gastric surgery, chronic concurrent illnesses (chronic kidney disease: serum creatinine >1.5 mg/dL; liver cirrhosis: Child-Pugh score B, C; and heart failure: ejection fraction <20%), or pregnancy were excluded due to concerns related to abnormal drug action or metabolism. Subjects administered antibiotics, bismuth, or PPIs in the 8 weeks preceding the study were also excluded.
This study was approved by the Institutional Research Ethics Board of The Catholic University of Korea (VC2 2RISI0064) and is in accordance with the Declaration of Helsinki. Informed consent was obtained from all patients.
Clarithromycin-resistant strains were identified in the tissue samples of the TT group using the CLO® test kit. DNA was extracted from the specimen using a QIAamp DNA Mini kit (Qiagen, Valencia, CA, USA) according to the manufacturer’s protocol and used for PCR amplification. DNA was stored at -20ºC until use. DPO-based multiplex-PCR (Seeplex® ClaR-H. pylori ACE detection; Seegene Institute of Life Science, Seoul, Korea) was performed to identify point mutation-containing gene fragments, according to the manufacturer’s recommendation. The kit included three primer pairs with a DPO structure that facilitated the amplification of H. pylori 23S rDNA. The single 621-bp DNA product represented wild-type H. pylori strain alone. DNA band at 475-bp or 194-bp indicated the presence of A2142G or A2143G mutation, respectively [15].
Two gastroenterologists participated in this study: one prescribed TT based on pretreatment clarithromycin resistance, and the other prescribed CT. In the TT group, patients without A2142G or A2143G mutation were treated with STT (lansoprazole 30 mg [Jeil Pharmaceutical Co., Seoul, Korea], amoxicillin 1 g [Chong Kun Dang Parmaceutical Co., Seoul, Korea], and clarithromycin 500 mg [Klaricid; Abbott Laboratories, North Chicago, IL, USA] twice daily for 14 days), whereas those carrying the mutations were managed with bismuth add-on STT comprising tripotassium dicitrate bismuthate (DENOL Greencross Co., Seoul, Korea, 300 mg four times daily) and STT for 14 days. Patients in the CT group were treated with lansoprazole 30 mg, amoxicillin 1 g, and clarithromycin 500 mg twice daily, plus metronidazole 500 mg (CJ Health Care Co., Seoul, Korea) three times daily for 10 days. Successful eradication was defined as a negative 13C-urea breath test (13C-UBT) conducted at least 4 weeks after the completion of eradication therapy. Medications related to the false negative result of 13C-UBT, such as proton pump inhibitors or histamine H2 receptor antagonists, were discontinued before the test.
Eradication failure with first-line regimen led to intervention using second-line therapy. A classic bismuth-containing quadruple therapy (BQT: rabeprazole 20 mg [rabeone; HK InnoN Co., Seoul, Korea] twice daily, tripotassium dicitrate bismuthate 300 mg four times daily, metronidazole 500 mg thrice daily, and tetracycline 500 mg four times daily for 7 days) was used as second-line regimen. Successful eradication was confirmed by 13C-UBT conducted 4 to 6 weeks after completion of the treatment. When patients failed two sessions of H. pylori eradication treatment, a third-line rescue regimen comprising rabeprazole 20 mg, amoxicillin 1 g, and levofloxacin 250 mg (Jeil Parmaceutical Co.) twice daily was prescribed for 14 days. Successful eradication was assessed via 13C-UBT.
The eradication rate was evaluated via per-protocol (PP) analysis. Treatment compliance was assessed at the follow-up visit with a questionnaire carrying items of consumed/total medication pill counts. We checked self-assessment of baseline symptoms and occurrence of an eradication therapy associated new symptoms. Non-compliance was defined as taking less than 80% of the prescribed total medication checked after treatment completion. A subgroup analysis of the second-line and third-line eradication rates was also conducted. We investigated the comparison of eradication rates and adverse events between TT and CT group for primary outcomes.
All data were recorded with standard forms and analyzed with a computer. Student’s t-test was used to compare continuous variables in the TT and CT groups. Differences between dichotomous variables were evaluated with the chi-square test. The calculation was performed with Statistical Package for the Social Sciences (SPSS) software (version 25.0; IBM, Chicago, IL, USA). P-values less than 0.05 were considered statistically significant.
A total of 210 patients were enrolled, which included 105 patients undergoing TT with pretreatment clarithromycin resistance test and 105 patients managed with CT. The baseline clinical characteristics of these two groups are shown in Table 1.
The eradication rates of TT group were 89.2% (91/102) in the PP analysis, respectively. In the CT group, the eradication rates were 81.6% (85/103), respectively. There were no statistically significant differences in eradication between PP analyses (P=0.12) (Fig. 1). The frequency of clarithromycin resistance in the TT group was 32.4% (34/105), and the eradication rate of bismuth add-on STT was 76.5% (26/34). During the study, five patients were classified as non-compliant due to side effects and excluded from the PP analyses. In the CT group, two patients had poor compliance, and one was admitted due to severe vomiting and hypokalemia. In the TT group, three patients showed poor compliance, including two patients receiving STT who complained of skin eruption and hypoglycemic symptoms and one patient receiving bismuth add-on STT who complained of diarrhea.
In the TT group, 12 patients were treated with second-line BQT, and the infection in 10 patients was successfully eradicated (10/12, 83.3%). Subsequently, two patients were treated with third-line levofloxacin-based rescue regimen and completed the treatment successfully, with the infection eradicated. In the CT group, 17 patients received second-line BQT, and the H. pylori infection in 13 of them was successfully eradicated (13/17, 76.5%). Two of the four patients in the CT group with second-line eradication failure were treated with a third-line levofloxacin-based rescue regimen, and one patient successfully eradicated the infection (50%). There was no statistical difference in the eradication rates of second-line treatment between TT and CT groups (P=0.35).
During the administration of the eradication regimen, 14 patients (6.7%) reported the occurrence of an H. pylori eradication therapy associated new symptoms. Five patients (in group of TT) had moderate to severe adverse events, and three patients did not complete the eradication regimen; diarrhea (n=2), allergic reaction of skin (n=1), hypoglycemia (n=1) and bitter taste/nausea (n=1). Nine patients (in group of CT) had moderate to severe adverse events, and two patients did not complete the eradication regimen; diarrhea (n=4), vomiting (n=1) and bitter taste/nausea (n=4). Furthermore, one patient in group CT had admission for hypokalemia after severe vomiting. There was no statistical difference of frequencies of adverse events between TT and CT (P=0.26).
In this study, we compared the efficacy of TT based on bismuth add-on STT with CT as a first-line regimen for H. pylori eradication. In the per protocol analysis, the eradication rates were 89.2% and 81.6% in TT and CT groups, with no statistical differences between the two groups. The frequency of clarithromycin resistance-associated point mutation was 33.3%, and the eradication rate with bismuth add-on STT was 77.8% in this subgroup. The differences in compliance and side effects between the two groups were not statistically significant. Considering the data, the result is acceptable for both TT and CT for first-line therapy of H. pylori.
Clarithromycin resistance of H. pylori is primarily associated with point mutations in the 23S ribosomal RNA gene. Among the point mutations, A2143G and A2142G are the most frequently observed subtypes in 81~93% of clarithromycin-resistant strains. Recently, a DPO‐based multiplex PCR assay for detecting these point mutations was introduced with specific advantages compared with culture studies based on susceptibility testing. It is easy to perform and has a short turnaround time for pathogen identification and mutation detection. The superior efficacy of TT based on this method compared with empirical therapy has been reported [10,16]. In a recent study in Korea, authors evaluated the efficacy of TT based on DPO‐based multiplex PCR compared with empirical CT [17]. The clarithromycin-based triple therapy and metronidazole-based triple therapy were performed based on the result of the PCR test, and the overall eradication rates were more than 80%, not significantly different compared to the CT group. However, the eradication rates of both were suboptimal in point mutation-positive subjects. Additional analysis based on H. pylori culture and antibiotic susceptibility tests revealed that 25% of cases with positive point mutations and 17.6% resistant to clarithromycin confirmed via susceptibility tests were also resistant to metronidazole, suggesting that the dual resistance may lead to suboptimal efficacy of metronidazole-based therapy in subjects carrying point mutations. We did not check the susceptibility tests for metronidazole resistance in this study. But in the high clarithromycin resistance, overcoming dual clarithromycin/metronidazole resistance is needed for the patients with point mutation. Increasing the daily dosage or duration of metronidazole has been suggested for dual resistant strains, and combination of metronidazole and bismuth is widely used as rescue therapy.
Bismuth is a classic drug known to exhibit antibacterial activity but is not classified as an antibiotic [18]. Although the mechanism of the antibacterial effect of bismuth on H. pylori is not fully understood, evidence suggests that bismuth inhibits the adherence and growth of H. pylori synergistically with antibiotics. It is known that bismuth compounds have cytoprotective effects on the gastric mucosa and help ulcer healing by adhering. The anti-helicobacter activities of bismuth exert directly and indirectly by accumulating and damaging bacterial walls and bacterial plasma membrane, inhibiting adherence of H. pylori to the surface mucosa, inhibiting enzymes like urease, fumarase, alcohol dehydrogenase, and inhibiting adenosine triphosphate (ATP) synthesis [19,20]. It also gives a chance to overcome antibiotic resistance by maximizing the effect of other antibiotics [20]. No bismuth-resistant H. pylori strains have been reported so far [21,22]. Several studies said that bismuth added to STT maximized the therapeutic effect and reduced the emergence of antibiotic-resistant H. pylori strains. Thus, adding bismuth could be a reasonable and essential choice in the era of rapidly growing antibiotic resistance [20,23]. In a meta-analysis, bismuth add-on STT showed the possibility of achieving ideal eradication in regions with relatively high clarithromycin resistance.13 A recent prospective study showed acceptable total eradication rates of 87.9% and 90% in ITT and PP analyses, respectively, with bismuth add-on STT [14]. The frequency of clarithromycin resistance in the study population assessed via DPO-based multiplex PCR was 33.6%, and the eradication rate was 77.1% in clarithromycin-resistant subjects. This study suggested that the eradication rate of TT with bismuth add-on STT was comparable to CT as a first-line regimen for H. pylori eradication in regions with high clarithromycin resistance. This beneficial effect was significant and appears to overcome clarithromycin resistance.
Our study had several limitations. First, since this study was retrospective and not a randomized controlled trial, it was difficult to directly confirm the characteristics of the control group. In particular, clarithromycin resistance was not confirmed in the CT group, and thus it was impossible to assess the eradication rate of CT in clarithromycin-resistant subjects and unable to compare the efficacy of bismuth add-on STT group, CT group in this study, and previously reported studies. Although suboptimal, the eradication rate associated with bismuth add-on STT in this study was higher than that of metronidazole-based triple therapy in the previous study [17]. However, another Korean study reported highly effective eradication rates with CT (>90%), even in patients with point mutations [8]. Further prospective controlled studies are needed to verify the efficacy of bismuth add-on STT compared with CT and other agents used in the alternative first-line regimen. Second, it was a single-center study, and the subjects in this study may not be representative of all Korean patients who require eradication therapy due to the potential regional differences not only in the prevalence of H. pylori infection but also in antimicrobial susceptibility.
In conclusion, TT with bismuth add-on STT was as effective as CT in the overall eradication rate. Results of a prospective randomized controlled trial suggest that this therapy can be used as a first-line regimen for H. pylori eradication in clarithromycin-resistant cases, with ideal efficacy without additional exposure to antibiotics or the need for classic BQT as a valid second-line regimen.
Fig. 1.
Comparison between eradication rates of tailored therapy (TT) using bismuth added to standard triple therapy and concomitant therapy (CT). PPA, per protocol analysis.
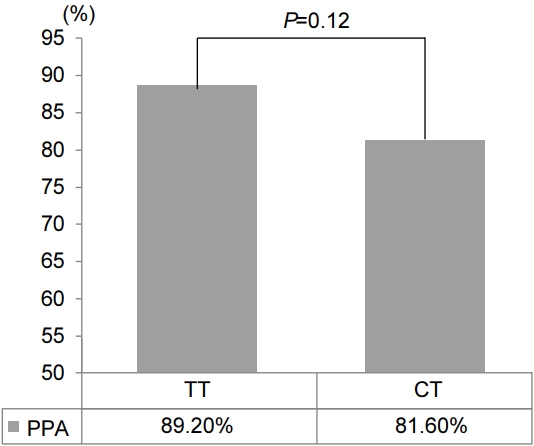
Table 1.
Clinical Characteristics of Tailored Therapy (TT) and Concomitant Therapy (CT) Groups
REFERENCES
1. Lee JW, Kim N, Kim JM, et al. Prevalence of primary and secondary antimicrobial resistance of Helicobacter pylori in Korea from 2003 through 2012. Helicobacter 2013;18:206-214.


2. Lee JH, Ahn JY, Choi KD, et al. Nationwide antibiotic resistance mapping of Helicobacter pylori in Korea: a prospective multicenter study. Helicobacter 2019;24:e12592.



3. Chey WD, Leontiadis GI, Howden CW, Moss SF. ACG clinical guideline: treatment of Helicobacter pylori infection. Am J Gastroenterol 2017;112:212-239.



4. Malfertheiner P, Megraud F, O'Morain CA, et al. Management of Helicobacter pylori infection-the Maastricht V/Florence consensus report. Gut 2017;66:6-30.


5. Yang JC, Lu CW, Lin CJ. Treatment of Helicobacter pylori infection: current status and future concepts. World J Gastroenterol 2014;20:5283-5293.



6. Mori H, Suzuki H. Update on quinolone-containing rescue therapies for Helicobacter pylori infection. World J Gastroenterol 2020;26:1733-1744.



7. Lee HJ, Kim JI, Lee JS, Jun EJ, et al. Concomitant therapy achieved the best eradication rate for Helicobacter pylori among various treatment strategies. World J Gastroenterol 2015;21:351-359.



8. Kim SY, Lee SW, Choe JW, et al. Helicobacter pylori eradication rates of concomitant and sequential therapies in Korea. Helicobacter 2017;22:e12441.


9. Lee BE, Kim JS, Kim BW, et al. Consistency of Helicobacter pylori eradication rates of first-line concomitant and sequential therapies in Korea: a nationwide multicenter retrospective study for the last 10 years. Helicobacter 2021;26:e12780.



10. Lee HJ, Kim JI, Cheung DY, et al. Eradication of Helicobacter pylori according to 23S ribosomal RNA point mutations associated with clarithromycin resistance. J Infect Dis 2013;208:1123-1130.


11. Jung HK, Kang SJ, Lee YC, et al. Evidence-based guidelines for the treatment of Helicobacter pylori infection in Korea: 2020 revised edition. Korean J Helicobacter Up Gastrointest Res 2020;20:261-287.


12. Cho JH, Jeon SR, Kim HG, Jin SY, Park S. Cost-effectiveness of a tailored Helicobacter pylori eradication strategy based on the presence of a 23S ribosomal RNA point mutation that causes clarithromycin resistance in Korean patients. J Gastroenterol Hepatol 2019;34:700-706.

13. Ko SW, Kim YJ, Chung WC, Lee SJ. Bismuth supplements as the first-line regimen for Helicobacter pylori eradication therapy: systemic review and meta-analysis. Helicobacter 2019;24:e12565.



14. Kim YJ, Chung WC, Kim DB. Efficacy of bismuth added to standard triple therapy as the first-line eradication regimen for Helicobacter pylori infection. Helicobacter 2021;26:e12792.



15. Woo HY, Park DI, Park H, et al. Dual-priming oligonucleotide-based multiplex PCR for the detection of Helicobacter pylori and determination of clarithromycin resistance with gastric biopsy specimens. Helicobacter 2009;14:22-28.


16. Francavilla R, Lionetti E, Castellaneta S, et al. Clarithromycin-resistant genotypes and eradication of Helicobacter pylori. J Pediatr 2010;157:228-232.


17. Ong S, Kim SE, Kim JH, et al. Helicobacter pylori eradication rates with concomitant and tailored therapy based on 23S rRNA point mutation: a multicenter randomized controlled trial. Helicobacter 2019;24:e12654.



19. Ge R, Chen Z, Zhou Q. The actions of bismuth in the treatment of Helicobacter pylori infections: an update. Metallomics 2012;4:239-243.


20. Dore MP, Lu H, Graham DY. Role of bismuth in improving Helicobacter pylori eradication with triple therapy. Gut 2016;65:870-878.


21. Alkim H, Koksal AR, Boga S, Sen I, Alkim C. Role of bismuth in the eradication of Helicobacter pylori. Am J Ther 2017;24:e751-e757.


-
METRICS

-
- 1 Crossref
- 2,218 View
- 69 Download
- Related articles in Korean J Helicobacter Up Gastrointest Res
-
Vonoprazan-based Dual and Triple Therapy for Helicobacter pylori Eradication2023 September;23(3)






